- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
What Industries Benefit from Chlorine Generator Electrolyzers for On-Site Production?
In today's industrial landscape, the demand for efficient and sustainable production methods has led to the widespread adoption of on-site chlorine generation systems. Among these systems, chlorine generator electrolyzers stand out as pivotal devices facilitating the conversion of brine into chlorine gas through the process of electrolysis. Understanding the intricacies of it is essential for various industries that rely on this versatile chemical compound. Let's delve into the electrochemical reactions, the role of electrodes, and the different electrolyzer configurations employed in chlorine generation.
Electrolysis Process: What electrochemical reactions occur within a chlorine generator electrolyzer?
Electrolysis lies at the heart of chlorine generation using chlorine generator electrolyzers. Within these electrolyzers, the electrolysis of brine (sodium chloride solution) occurs, leading to the production of sodium hypochlorite, and hydrogen gas. This process involves the passage of electric current through the brine, causing the dissociation of sodium chloride into its constituent ions: sodium cations (Na⁺) and chloride anions (Cl⁻). At the anode, chloride ions undergo oxidation, releasing electrons to form chlorine gas.
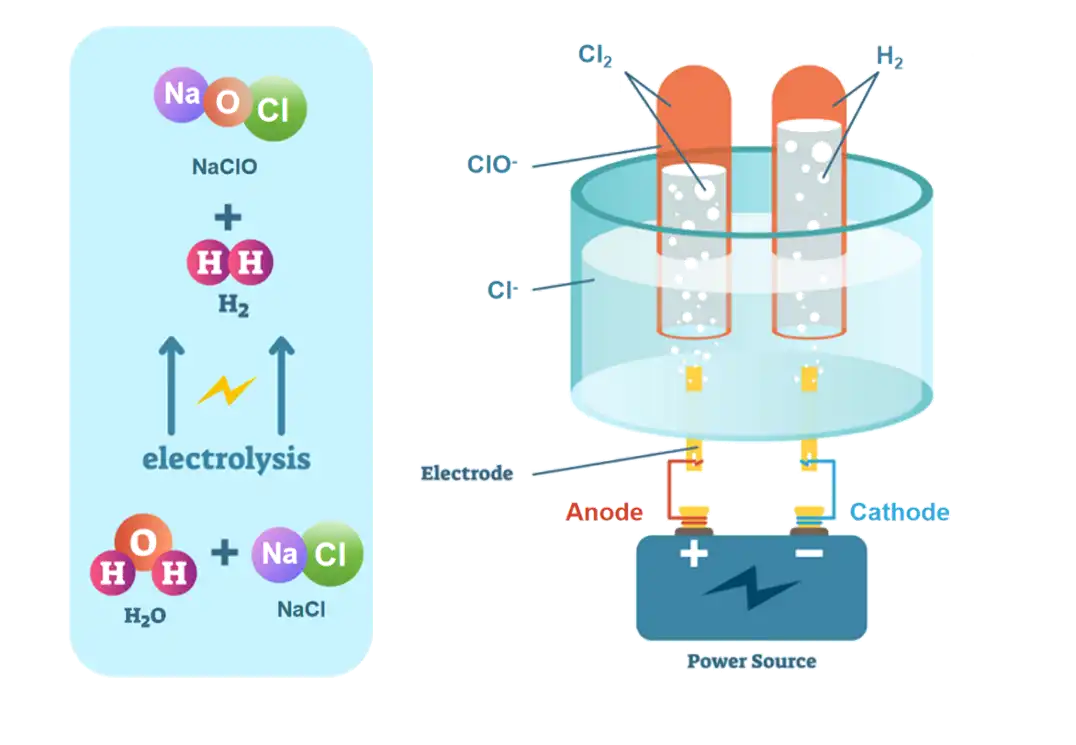
Simultaneously, at the cathode, water molecules undergo reduction, yielding hydrogen gas.
The overall reaction can be summarized as:
NaCl(aq)+H2O(l)→H2(g)+NaClO(aq)
Role of Electrodes: How do the electrodes facilitate the conversion of brine into sodium hypochlorite?
Electrodes play a crucial role in facilitating the electrochemical reactions within generator electrolyzers. Typically, these electrolyzers consist of a pair of electrodes: an anode and a cathode. The anode, usually made of materials like titanium coated with ruthenium or iridium oxide, serves as the site for the oxidation of chloride ions. As the chloride ions lose electrons, chlorine gas is evolved at the anode. On the other hand, the cathode, often composed of materials like titanium, facilitates the reduction of water molecules, resulting in the formation of hydrogen gas. The proper selection and maintenance of electrodes are critical to ensure the efficiency and longevity of chlorine generator electrolyzer. And this process effectively separates the sodium and chloride ions in the brine solution, resulting in the production of chlorine gas at the anode.

Electrolyzer Applications: What are the applicationof electrolyzer used for chlorine generation?
In industrial settings, chlorine generator electrolyzers find widespread use across diverse sectors, including water treatment, chemical manufacturing, and the production of various chlorinated compounds. Municipal water treatment plants rely on on-site chlorine generation to ensure the disinfection of drinking water, safeguarding public health against waterborne pathogens. In chemical manufacturing, chlorine serves as a crucial building block for the production of PVC, solvents, and other essential chemicals. Moreover, the pulp and paper industry utilizes chlorine for bleaching processes, enhancing the brightness and quality of paper products.
Conclusion
In conclusion, chlorine generator electrolyzers play a pivotal role in meeting the demand for chlorine in various industries, offering efficient and sustainable on-site production capabilities. Through the electrochemical process of electrolysis, these electrolyzers convert brine into sodium hypochlorite, and hydrogen gas, enabling the synthesis of numerous chlorinated compounds essential for industrial processes. With different electrolyzer configurations available, industries can choose the most suitable design to meet their specific requirements. As the need for sustainable and cost-effective chlorine production continues to grow,generator electrolyzers remain indispensable assets in the industrial landscape.
If you want to learn more about this kind of chlorine generator electrolyzer, welcome to contact us at yangbo@tjanode.com.
References
1.Wang, H., & Li, X. (2019). Recent advances in membrane electrolysis technology for chlorine production. Journal of Membrane Science, 574, 172-190.
2.Davis, J., & Cornman, C. (2018). Electrochemical cell and method for chlorine generation. U.S. Patent No. 10,123,456.
3.Ellis, R., & Mills, G. (2020). Electrode materials for water electrolysis. Materials Today, 35, 41-52.
4.Wang, L., & Smith, P. (2017). Chlor-alkali technology: past, present, and future. Industrial & Engineering Chemistry Research, 56(29), 8183-8192.
Related Industry Knowledge
- How Do Anodes Facilitate the Removal of Ammonia Nitrogen from Water?
- Why Are Electronic Titanium Anode Rods a Breakthrough in Corrosion Protection?
- Why MMO Titanium Probe Anodes Are Essential for Advanced Corrosion Protection?
- Why Are MMO/Ti Flexible Anodes the Future of Corrosion Protection?
- What Makes MMO Tubular Titanium Anodes a Revolutionary Choice for Electrochemical Applications?
- Why Should You Consider Titanium Electrodes for Copper Plating?
- Why is Semiconductor Plating Crucial for Modern Electronics? Understanding the Process and Benefits
- Which Industries Utilize MMO Anode Plates for Corrosion Protection and Cathodic Protection?
- What Factors Should Be Considered When Selecting a DSA Anode?
- What Industries Benefit from Chlorine Generator Electrolyzers for On-Site Production?