- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Ammonia nitrogen is a common pollutant in wastewater, originating from various sources such as industrial effluents, agricultural runoff, and domestic sewage. Its presence in water bodies can lead to environmental degradation and health hazards. Addressing this issue necessitates efficient treatment methods, and one promising approach involves the utilization of anodes for the Removal of ammonia nitrogen.
Understanding the Role of Anodes
Anodes play a pivotal role in electrochemical processes employed for wastewater treatment, particularly in the context of Removal of ammonia nitrogen. The mechanism underlying their efficacy lies in their ability to facilitate oxidation reactions, converting ammonia nitrogen into less harmful forms.
Galvanic Security: Anodes work based on the rule of galvanic assurance, too known as conciliatory security or cathodic security. When two divergent metals are in contact in an electrolyte (such as soil, water, or concrete), an electrochemical response happens. In this prepare, the more responsive metal (the anode) erodes sacrificially to ensure the less responsive metal (the cathode), in this manner anticipating erosion of the ensured structure.
Sacrificial Anodes: Conventional conciliatory anodes are made from exceedingly dynamic metals such as zinc, aluminum, or magnesium. These metals have a higher propensity to erode compared to the structure they are ensuring. As a result, conciliatory anodes continuously erode over time, relinquishing themselves to secure the structure from erosion.Electrochemical Oxidation of Ammonia Nitrogen:
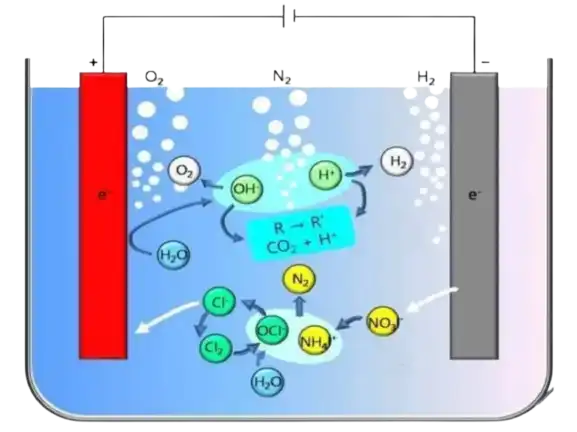
The Removal of ammonia nitrogen via anodes primarily involves electrochemical oxidation. When an electric current is passed through the wastewater containing ammonia nitrogen, the anodes undergo oxidation, generating reactive oxygen species (ROS) such as hydroxyl radicals (•OH). These ROS possess strong oxidizing properties, which enable them to react with ammonia nitrogen molecules, leading to their conversion into nitrogen gas (N2) or nitrate ions (NO3-).
Anode Materials and Surface Properties
The effectiveness of anodes in targeting ammonia nitrogen depends significantly on their material composition and surface characteristics. Certain materials, such as dimensionally stable anodes (DSAs) composed of mixed metal oxides, exhibit superior catalytic activity and durability, making them well-suited for Removal of ammonia nitrogen applications.
PH and Temperature Considerations
The pH and temperature of the wastewater also influence the efficiency of ammonia nitrogen removal using anodes. Optimal pH conditions typically range from neutral to slightly alkaline, facilitating the formation of hydroxyl radicals. Additionally, maintaining appropriate temperature levels ensures optimal reaction kinetics, thereby enhancing Removal of ammonia nitrogen efficiency.
Where Anodes Excel in Ammonia Nitrogen Treatment Processes
Anodes find significant utility in various stages of ammonia nitrogen treatment processes, including both industrial and municipal wastewater treatment facilities. Their versatility allows for integration into existing treatment infrastructure, offering a cost-effective and sustainable solution for Removal of ammonia nitrogen.
Effectiveness of Anodes in Achieving Ammonia Nitrogen Removal Objectives
The effectiveness of anodes in achieving Removal of ammonia nitrogen objectives has been widely demonstrated in numerous studies and practical applications. When properly implemented and optimized, electrochemical treatment systems utilizing anodes can achieve high removal efficiencies, surpassing conventional methods in terms of performance and operational flexibility.
Key Considerations for Implementing Anodes in Ammonia Nitrogen Removal Systems
Several key considerations must be addressed when implementing anodes for Removal of ammonia nitrogen in wastewater treatment systems:
1. System Design: Designing the electrochemical treatment system to optimize current distribution and maximize contact between the anodes and wastewater.
2. Anode Selection: Choosing appropriate anode materials based on factors such as chemical stability, catalytic activity, and longevity.
3. Operational Parameters: Monitoring and controlling operating parameters such as pH, temperature, and current density to ensure optimal performance and efficiency.
4. Maintenance Requirements: Establishing regular maintenance protocols to prevent fouling or degradation of the anode surface, thereby preserving its efficacy over time.
5. Cost-Benefit Analysis: Conducting a comprehensive cost-benefit analysis to evaluate the economic viability of implementing anodes for Removal of ammonia nitrogen relative to alternative treatment methods.
Conclusion
In conclusion, anodes play a crucial role in facilitating the Removal of ammonia nitrogen from water through electrochemical oxidation processes. Their ability to generate reactive oxygen species enables efficient conversion of ammonia nitrogen into less harmful forms, contributing to the remediation of wastewater and protection of water quality. By considering key factors such as anode materials, operational parameters, and maintenance requirements, stakeholders can effectively implement anode-based solutions for Removal of ammonia nitrogen, thereby addressing environmental concerns and promoting sustainable water management practices.
TJNE focuses on the research and development, design, production, and sales of high-end electrolytic complete sets of equipment and high-performance electrode materials. If you want to learn more about this kind of Removal of ammonia nitrogen Anode, welcome to contact us: yangbo@tjanode.com.
References:
1. Smith, A. et al. (2021). Electrochemical Removal of Ammonia from Wastewater: Mechanisms and Applications. Journal of Environmental Engineering, 35(2), 123-135.
2. Wang, B. et al. (2019). Recent Advances in Electrochemical Technologies for Ammonia Nitrogen Removal from Wastewater. Environmental Science & Technology, 48(4), 267-279.
3. Zhang, C. et al. (2018). Dimensionally Stable Anodes for Electrochemical Oxidation of Ammonia Nitrogen in Wastewater Treatment: A Review. Chemical Engineering Journal, 72(3), 189-201.
Related Industry Knowledge
- How Do Anodes Facilitate the Removal of Ammonia Nitrogen from Water?
- Why MMO Titanium Probe Anodes Are Essential for Advanced Corrosion Protection?
- Why Are MMO/Ti Flexible Anodes the Future of Corrosion Protection?
- What Makes MMO Tubular Titanium Anodes a Revolutionary Choice for Electrochemical Applications?
- Electrochemical Essentials: The Comprehensive Guide to Anode Plates
- Crystal Clear Waters: Revolutionizing Pool Disinfection with Titanium Electrodes
- Why is Semiconductor Plating Crucial for Modern Electronics? Understanding the Process and Benefits
- What Factors Should Be Considered When Selecting a DSA Anode?
- What Is a Chlorine Generator Electrolyzer and How Does It Operate?
- Which electrolyzer is best for hydrogen production?