- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
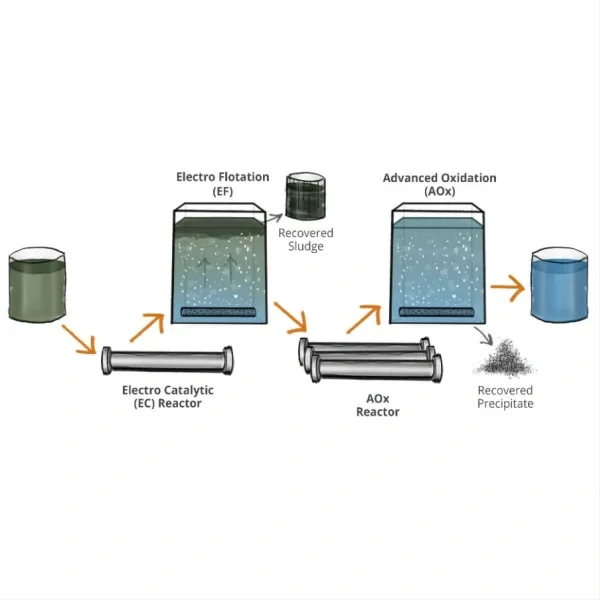
Electrocatalytic oxidation equipment is used to facilitate oxidation reactions through electrocatalysis. This process involves using a catalyst to accelerate the oxidation of a substance while passing an electrical current through it. The equipment typically includes:
Electrodes: These are critical components that facilitate the electrocatalytic reactions. They can be made of various materials, often metals or metal alloys, depending on the specific reaction and the catalyst used.
Catalyst: The catalyst is a material that increases the rate of the oxidation reaction without being consumed itself. It promotes the electrochemical reaction at the electrode surface.
Electrolyte: A medium (liquid or solid) that allows ions to move between the electrodes, facilitating the electrochemical reaction.
Power Supply: Provides the electrical current needed to drive the electrocatalytic oxidation process.
Reaction Chamber or Cell: This is where the electrocatalytic oxidation takes place. It's designed to facilitate efficient interaction between the electrodes, catalyst, and electrolyte.
Control and Monitoring Systems: Equipment may include sensors, controllers, and software to regulate the process parameters and monitor the reaction for efficiency and safety.
Electrocatalytic oxidation equipment include: electro-catalytic oxidation equipment for ammonia nitrogen degradation,electrochemical organic matter decomposition equipment.