- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Electrochemical equipment encompasses various tools and devices used in electrochemistry, a branch of science dealing with the interaction between electrical and chemical processes. This equipment is designed to facilitate and study chemical reactions involving electricity. Some common components and instruments include:
Potentiostat/Galvanostat: An essential instrument used to control voltage (potentiostat) or current (galvanostat) during electrochemical experiments. It helps in applying precise potentials or currents to the working electrode.
Electrodes: These are crucial components that come in various types such as reference electrodes, working electrodes, and counter electrodes. They facilitate the electrochemical reaction by either generating or consuming electrons.
Electrolyte Solutions: Solutions containing ions that facilitate the movement of charge between electrodes during the electrochemical process.
Electrochemical Cells: These cells are setups where the electrochemical reactions occur. They can be divided into various types such as two-electrode cells, three-electrode cells, etc., based on their configurations.
Electrochemical Analyzers: Instruments used for analyzing and measuring the electrochemical behavior of substances. They often include capabilities for voltammetry, amperometry, impedance spectroscopy, and other electrochemical techniques.
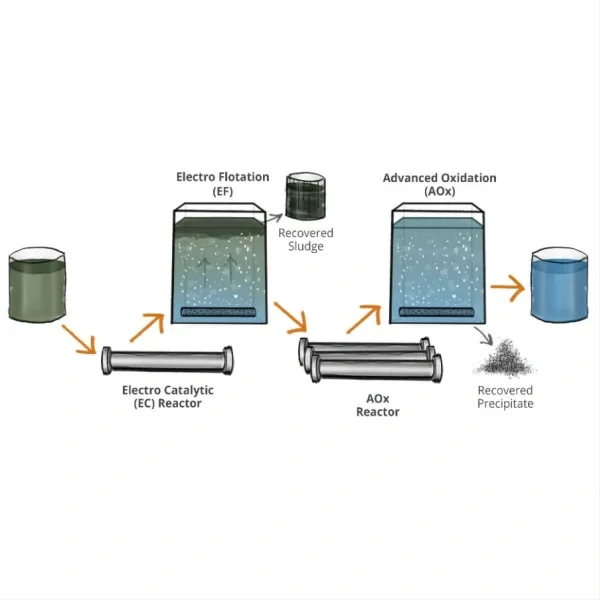
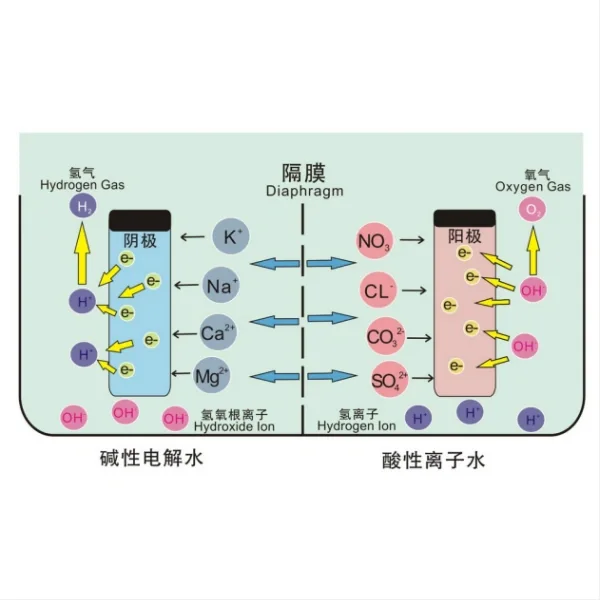
Electrochemical equipment include: high efficiency copper dissolution tank,copper foil anode,titanium anode tank,copper foil surface treatment machine,electro-catalytic oxidation equipment for ammonia nitrogen degradation,electrochemical organic matter decomposition equipment,electrode-diaphragm assembly for alkaline water electrolysis,polymer electrolyte membrane(pem)electrolyzers,nel alkaline electrolyser,ion membrane electrolyzer,high concentration sodium hypochlorite generator (diaphragm electrolysis),nacl diaphragm electrolyzer.