- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
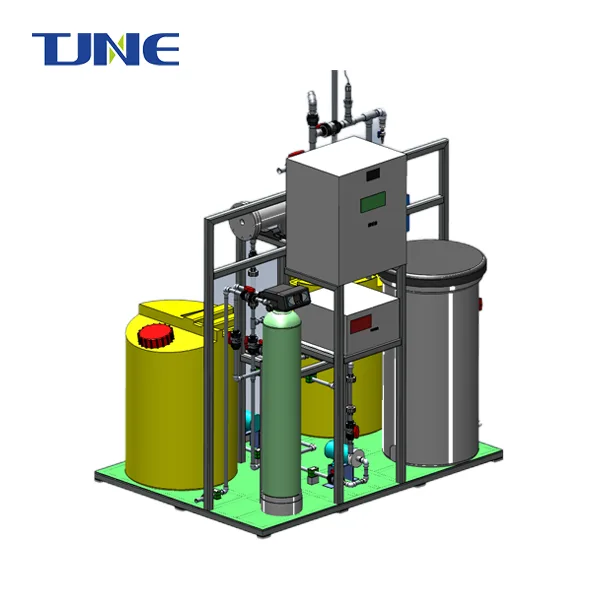
Brine Electrolysis Equipment
Product Overview: It is a device that uses electrolysis technology to separate chloride ions and sodium ions in brine.
Product composition: electrolyzer, power supply, electrolyte circulation system, gas collection system, etc.
Product advantages: It has the advantages of high efficiency, environmental protection, energy saving, and small equipment footprint.
Application fields: used in chemical industry, pharmaceutical, water treatment and other fields.
Product after-sales service: We provide timely, high-quality new anode manufacturing and old anode recoating services worldwide.
Brine Electrolysis Equipment Product Details:
Brine Electrolysis Equipment is a pivotal element of colorful artificial processes that bear the product of high- chastity chlorine gas, similar to the manufacturing of chemicals, petrochemicals, and water disinfection. The outfit is designed to efficiently convert sodium chloride results into chlorine gas and other precious derivations.
Brine electrolysis is a chemical process that uses an electrolytic cell to separate sodium chloride into its constituent rudiments chlorine gas, acidulous soda pop( sodium hydroxide), and hydrogen gas. The process takes place in a Brine electrolysis outfit, which consists of several crucial factors, including an anode, a cathode, and a diaphragm or membrane.
Working Principle:
The Brine Elelectrolysis outfit uses direct current( DC) to drive the electrochemical responses. Sodium ions resettle towards the cathode, where they're reduced to sodium essence, while chloride ions move towards the anode, where they're oxidized to chlorine gas. The diaphragm or membrane separates the two electrodes, precluding the mixing of chlorine gas with acidulous soda pop and hydrogen gas. The chlorine gas is collected and purified for further use, while the acidulous soda pop and hydrogen gas are independently captured and employed in colorful artificial processes.
System Components and Features:
The brine electrolysis equipment consists of the following components:
-
Anode: Made of a suitable material (such as titanium) that can withstand the corrosive environment.
-
Cathode: Typically made of nickel or stainless steel, providing a high level of electrical conductivity.
-
Diaphragm/Membrane: Separates the anode and cathode compartments, ensuring the efficient separation of chlorine gas from other byproducts.
-
Electrolyte Circulation System: Facilitates the continuous flow of brine solution through the electrolytic cell to maintain the required concentration.
-
Gas Collection System: Collects and purifies chlorine gas for safe storage and future use.
Performance Parameters:
The brine electrolysis equipment offers the following performance parameters:
| Parameter | Value |
|---|---|
| Chlorine Production | Up to 1000 kg/h |
| Sodium Hydroxide Concentration | Up to 50% |
| Hydrogen Production | Up to 1.5 kg/h |
| Energy Consumption | Less than 3 kWh/kg chlorine |
Technical Specifications:
The brine electrolysis equipment meets the following technical specifications:
| Specification | Value |
|---|---|
| Operating Voltage | 3-5 V |
| Operating Temperature | 50-80°C |
| Electrolyte Flow Rate | 10-50 L/min |
Economic Indicators:
The brine electrolysis equipment offers the following economic indicators:
| Indicator | Value |
|---|---|
| Return on Investment (ROI) | Within 2 years |
| Payback Period | Within 12-18 months |
Key Features and Advantages:
-
High chlorine production capacity.
-
Efficient separation of chlorine gas from other byproducts.
-
Low energy consumption.
-
Long lifespan and low maintenance requirements.
-
Flexible operating parameters to meet specific production needs.
Applications:
The brine electrolysis equipment is widely used in the following industries:
-
Chemical manufacturing.
-
Petrochemical processing.
-
Water disinfection.
FAQ:
Q: Can the brine electrolysis equipment be customized for specific production requirements?
A: Yes, we offer customized solutions based on the specific needs of our customers.
Q: What is the typical lifespan of the equipment?
A: The equipment has a long lifespan of over 15 years with proper maintenance.
Q: How is the chlorine gas collected and stored?
A: The chlorine gas is collected using a gas collection system and stored in appropriate containers under safe conditions.
Contact Us
If you are interested in selecting your own Brine Electrolysis Equipment, please feel free to contact us at yangbo@tjanode.com. We are a professional manufacturer and supplier of Brine Electrolysis Equipment, offering strong technical expertise, one-stop after-sales service, complete certification and testing reports, fast delivery, secure packaging, and support for inspection.