- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
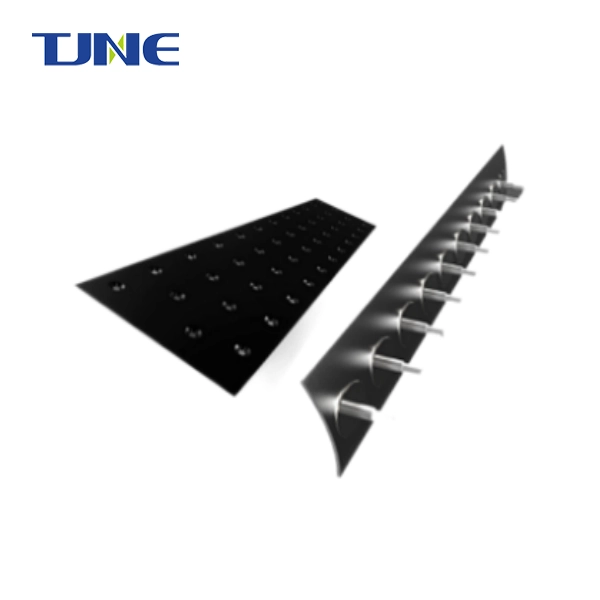
A DSA Anode, also known as a Dimensionally Stable Anode, is an anode extensively used in various electrochemical processes. It is designed to provide exceptional durability and resistance to corrosion, enabling consistent performance over extended periods. One of their key qualities is their capacity to keep up with layered security during electrochemical responses. In contrast to conventional anodes, they may degrade or dissolve over time, maintain their shape and structural integrity, ensuring long-term dependability and minimizing the risk of contamination. They offer a few benefits over traditional anodes. They display high current thickness abilities, taking into account productive electrochemical responses. They additionally add to energy reserve funds by diminishing power utilization during electrolytic cycles.
Structure and Composition: What materials are typically used in the construction of DSA anodes?
DSA (Dimensionally Stable Anode) anodes represent a remarkable advancement in electrochemical technology, offering durability and efficiency in various industrial processes. These anodes are constructed using a combination of materials carefully selected to withstand harsh operating conditions while maintaining stability over extended periods. Typically, DSA anodes consist of a substrate material coated with a thin layer of catalytically active material.
The substrate material, often titanium or its alloys, provides the structural integrity necessary to withstand corrosive environments and mechanical stress. Titanium's excellent corrosion resistance, coupled with its strength and light weight, makes it an ideal choice for the substrate of it. Additionally, noble metals such as ruthenium, iridium, or platinum are commonly employed as catalytic coatings due to their high activity and stability in electrochemical reactions.
Electrochemical Process: How does a DSA anode facilitate electrochemical reactions?
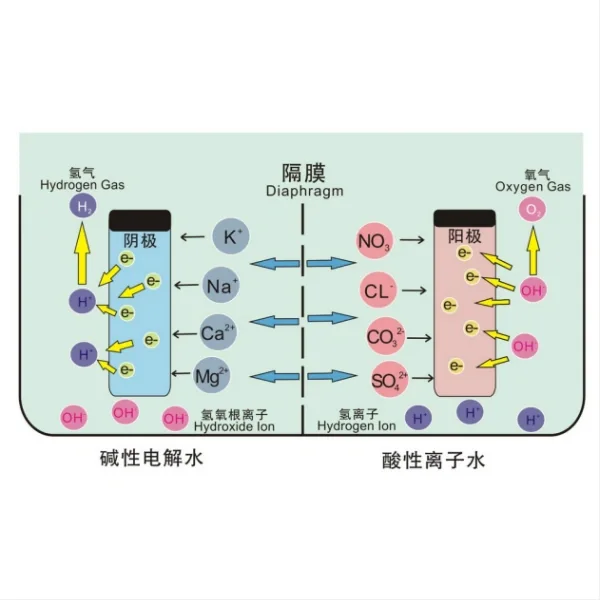
This anode operates based on the principle of electrocatalysis, where the catalytic coating facilitates desired electrochemical reactions while minimizing side reactions such as oxygen evolution or metal dissolution. This process occurs at the interface between the anode surface and the electrolyte solution.
When a potential is applied across the DSA anode-electrolyte interface, electrochemical reactions are initiated. The catalytic coating on the anode surface provides active sites for specific reactions to take place, effectively reducing the activation energy required for these reactions to occur. For instance, in electrochemical processes such as water electrolysis or electrochemical oxidation, the catalytic coating promotes the desired reactions while inhibiting the undesired reactions that could lead to degradation of the anode material.
It exhibits dimensional stability during operation, meaning they maintain their structural integrity without undergoing significant changes in size or shape. This stability is crucial for ensuring consistent performance and prolonging the lifespan of the anode in various industrial applications.
Operating Principles: What factors influence the performance of a DSA anode?
Several factors influence the performance of this anodes, ranging from design considerations to operating conditions. Understanding and optimizing these factors are essential for maximizing the efficiency and longevity of DSA anode-based systems.
1.Catalyst Selection:
The choice of catalytic coating greatly influences the efficiency and selectivity of electrochemical reactions. Factors such as catalytic activity, stability, and compatibility with the operating environment must be carefully considered during catalyst selection.
2.Surface Morphology:
The morphology of the anode surface plays a crucial role in facilitating mass transport and ensuring uniform current distribution. Optimizing surface roughness and porosity can enhance the accessibility of active sites and improve the overall performance of it.
3.Operating Conditions:
Parameters such as temperature, pH, and electrolyte composition significantly impact the electrochemical performance of this anode. Maintaining optimal operating conditions is essential for achieving desired reaction rates while minimizing degradation of the anode material.
4.Current Density:
The applied current density directly affects the rate of electrochemical reactions occurring at the its surface. Balancing current density to avoid overloading or underutilizing the anode is critical for maximizing efficiency and preventing premature failure.
5.Mechanical Stability:
Ensuring mechanical stability is vital for preventing delamination or physical damage to the catalytic coating during operation. Proper substrate selection and coating techniques can enhance the mechanical robustness of them, improving their long-term performance and reliability.
Contact Us
In conclusion, DSA anodes represent a sophisticated solution for various electrochemical applications, offering durability, efficiency, and dimensional stability. By carefully selecting materials, optimizing design parameters, and controlling operating conditions, the performance of DSA anodes can be maximized to meet the demands of diverse industrial processes.
If you want to learn more about it, welcome to contact us at yangbo@tjanode.com.
References
1.Bockris, J. O'M., & Reddy, A. K. N. (1970). Modern Electrochemistry: An Introduction to an Interdisciplinary Area. Springer.
2.Comninellis, C. (1994). Electrocatalysis in the Anodic Evolution of Oxygen and Chlorine. Journal of Applied Electrochemistry, 24(11), 1077–1085. doi:10.1007/bf00249644
3.Conway, B. E. (1999). Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications. Springer.
4.Groysman, A. (2010). Electrochemical Aspects of Ionic Liquids. Wiley-VCH.
5.Srinivasan, S., & Ticianelli, E. A. (Eds.). (2007). Advances in Direct Methanol Fuel Cells. Springer.
Related Industry Knowledge
- Electrochemical Evolution: The Advanced Applications of MMO Belts
- The Protective Power of MMO Ribbon Anodes: A Deep Dive into Cathodic Protection
- Crystal Clear Waters: The Power of Titanium Electrodes in Pool Disinfection
- Electrochemical Innovation: The Role of Iridium-Tantalum Coated Titanium Anodes
- Revolutionizing Industries: The Power of Ruthenium-Iridium Coated Titanium Anodes
- The Power of Splitting Water: An In-Depth Look at Alkaline Water Electrolyzers
- What Advantages Do Electrodeposited Titanium Electrodes Offer for Cobalt Plating?
- How Does PCB VCP DC Copper Plating Work in Direct Current Systems?
- What is a DSA Anode and How Does It Work?
- What Industries Benefit from Chlorine Generator Electrolyzers for On-Site Production?