- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
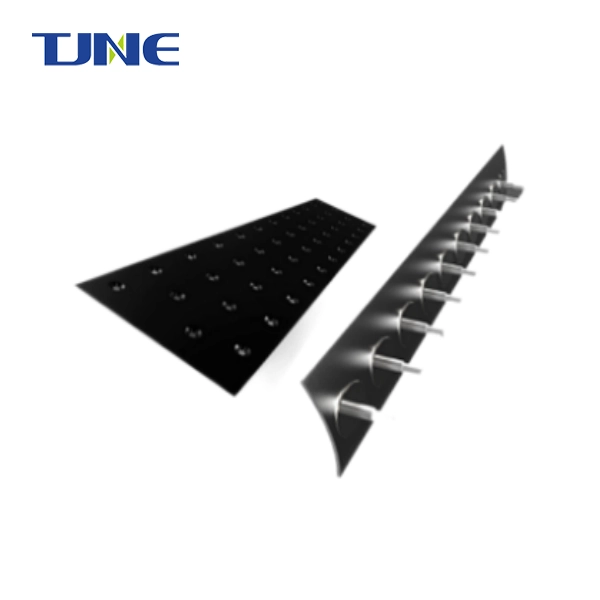
What Is an MMO Anode Plate and How Does It Function in Electrochemical Processes?
In the realm of electrochemical processes, MMO anode plates stand as essential components facilitating numerous applications. These plates, crafted from a blend of metal and metal oxide, exhibit remarkable properties crucial for their functionality. Comprising titanium as the primary metal and ruthenium oxide as the oxide component, they boast a robust structure tailored to withstand harsh environments and demanding operational conditions.
Composition and Structure
Titanium, renowned for its exceptional corrosion resistance and mechanical strength, serves as the backbone of them. This metal's inherent durability enables the plates to endure prolonged exposure to corrosive substances and high current densities without succumbing to degradation. Moreover, titanium's lightweight nature facilitates ease of installation and maintenance, making the anode plates highly practical for various electrochemical applications.
Augmenting the titanium matrix, ruthenium oxide infuses the anode plates with enhanced electrochemical performance. Ruthenium oxide possesses catalytic properties crucial for promoting desired electrochemical reactions while maintaining stability over extended periods. Through its synergistic interaction with titanium, ruthenium oxide optimizes the efficiency and longevity of them, ensuring consistent performance throughout their operational lifespan.
Electrochemical Reaction
MMO anode plates play a pivotal role in facilitating electrochemical reactions across diverse industries, ranging from water treatment to metal electroplating. Central to their functionality is their ability to act as catalysts, expediting desirable reactions while inhibiting undesirable ones. In electrolytic processes, such as water disinfection or metal recovery, they promote the generation of oxygen and chlorine gas through the electrolysis of water or brine solutions.
During electrolysis, a direct current passes through the anode plates, inducing oxidation reactions at their surface. At the anode, water molecules undergo electrolysis, yielding oxygen gas at the anode plate's surface while releasing protons. Simultaneously, chloride ions present in the electrolyte solution undergo oxidation, resulting in the liberation of chlorine gas. These reactions occur selectively at the anode plate, driven by the catalytic properties of ruthenium oxide, thereby facilitating the desired electrochemical transformations.
Furthermore, they find application in cathodic protection systems, safeguarding metallic structures against corrosion. By serving as sacrificial anodes, the anode plates ensure the preferential corrosion of their surface, preserving the integrity of the protected structure. This mechanism relies on their ability to sustain high current densities without undergoing detrimental corrosion, thus effectively shielding the protected structure from the corrosive environment.
Corrosion Resistance
MMO anode plates are widely recognized for their exceptional corrosion resistance in various electrochemical applications. The key to their superior performance lies in the composition of the mixed metal oxide coating applied to the substrate material, typically titanium. This coating forms a protective passive film when exposed to electrolytes, effectively shielding the substrate from corrosive elements and enhancing the overall corrosion resistance of the anode plates.
One of the significant advantages of them is their high resistance to chloride-rich environments, which makes them ideal for applications such as chlor-alkali production, seawater electrolysis, and swimming pool water treatment. In these environments, other types of anode plates would suffer from rapid degradation due to chlorine-induced corrosion, but they can provide reliable and long-lasting performance.
In addition to their excellent resistance to chloride, these anode plates exhibit outstanding resistance to acidic conditions, making them suitable for use in industries where acid solutions are present. Their corrosion-resistant properties protect the substrate material from degradation and ensure prolonged service life.
Moreover, they can withstand high temperatures without compromising their corrosion-resistant properties, providing reliability in applications that involve high operating temperatures. This stability enables the anode plates to withstand thermal stress and maintain their protective properties under challenging conditions.
The exceptional corrosion resistance of our product contributes to their longevity and durability. By withstanding corrosive environments and minimizing degradation, they can operate efficiently for extended periods without the need for frequent replacements. This prompts a decrease in support necessities and margin time, bringing about cost reserve funds and improved functional proficiency.
Overall, they are a reliable and cost-effective choice for industries that demand durable and corrosion-resistant electrode materials. Their ability to resist degradation in harsh environments makes them an ideal solution for various electrochemical applications.
Conclusion
In conclusion, MMO anode plates represent indispensable components in electrochemical processes, owing to their robust composition, catalytic properties, and exceptional corrosion resistance. By harnessing the synergistic interplay between titanium and ruthenium oxide, these plates enable efficient electrochemical reactions while withstanding the rigors of demanding operational conditions. From water treatment to cathodic protection, anode plates exemplify reliability, durability, and performance in diverse applications.
If you want to learn more about this kind of MMO Anode Plate, welcome to contact us: yangbo@tjanode.com.
References
1. Wang, L., & Zhang, S. (2018). Titanium-based shape memory alloys. Materials Science and Engineering: R: Reports, 132, 1-52.
2. Pan, C., Xu, Q., Zheng, Y., et al. (2020). Recent advances in the application of titanium dioxide nanotube arrays in photoelectrochemical water splitting. Journal of Materials Chemistry A, 8(22), 10958-10985.
3.Zeng, K., & Zhang, D. (2008). Recent progress in alkaline water electrolysis for hydrogen production and applications. Progress in Energy and Combustion Science, 34(4), 470-490.
4.Ali, N., Qamar, M., & Ali, N. (2021). A comprehensive review on electrochemical sensors based on ruthenium oxide for the detection of hazardous substances. Microchemical Journal, 161, 105754.
5.Rajkumar, P., & Kim, Y. (2020). Electrochemical aspects of ruthenium oxide thin film electrodes: a review. Journal of Solid State Electrochemistry, 24(10), 2557-2570.
Related Industry Knowledge
- Why MMO Titanium Probe Anodes Are Essential for Advanced Corrosion Protection?
- Electrochemical Evolution: The Advanced Applications of MMO Belts
- The Protective Power of MMO Ribbon Anodes: A Deep Dive into Cathodic Protection
- How Does Using Electrodeposited Titanium Electrodes Transform Zinc Plating Processes?
- Why Should You Consider Titanium Electrodes for Copper Plating?
- How Does a Chlorine Generator Electrolyzer Enhance Pool Maintenance?
- What Makes PCB RPP Copper Plating Essential for Durable Surface Area Enhancement?
- How Does PCB VCP DC Copper Plating Work in Direct Current Systems?
- What Is an MMO Anode Plate and How Does It Function in Electrochemical Processes?
- What Factors Should Be Considered When Selecting a Chlorine Generator Electrolyzer System?