- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Saltwater electrolysis is a crucial process in various industries, from water treatment to hydrogen production. At the heart of this electrochemical reaction are the electrodes, which play a pivotal role in the efficiency and effectiveness of the process. Among the different materials available for electrodes, titanium has emerged as a frontrunner, offering numerous advantages that make it an ideal choice for saltwater electrolysis. This blog post will delve into the benefits of using titanium electrodes and explore some of the most frequently asked questions about their application in saltwater electrolysis.
Why are titanium electrodes preferred for saltwater electrolysis?
Titanium electrodes have become the material of choice for saltwater electrolysis due to their unique combination of properties that make them exceptionally well-suited for this demanding application. The preference for titanium in this context stems from several key factors that contribute to its superior performance and longevity in corrosive saltwater environments.
First and foremost, titanium's exceptional corrosion resistance is a primary reason for its popularity in saltwater electrolysis. When exposed to saltwater, titanium forms a stable, protective oxide layer on its surface. This naturally occurring phenomenon, known as passivation, effectively shields the underlying metal from further corrosion. Unlike many other metals that degrade rapidly in saltwater, titanium's passive film remains intact even under the harsh conditions of electrolysis, ensuring the electrode's longevity and consistent performance over extended periods.
The durability of titanium electrodes translates directly into cost-effectiveness for industrial applications. While the initial investment in titanium electrodes may be higher compared to some alternatives, their extended lifespan and minimal maintenance requirements result in significant long-term savings. Facilities using titanium electrodes can operate for longer periods without interruptions for electrode replacement or repairs, leading to increased productivity and reduced downtime.
Another crucial advantage of titanium electrodes is their high electrical conductivity. Despite forming a protective oxide layer, titanium maintains excellent electrical properties, allowing for efficient electron transfer during the electrolysis process. This characteristic is vital for maximizing the energy efficiency of saltwater electrolysis systems, as it minimizes resistance and reduces power consumption.
Titanium's mechanical strength is yet another factor that contributes to its preference in saltwater electrolysis. The electrodes must withstand not only the corrosive environment but also the physical stresses associated with gas evolution and fluid dynamics within the electrolysis cell. Titanium's robust nature ensures that the electrodes maintain their structural integrity under these demanding conditions, reducing the risk of mechanical failure and enhancing overall system reliability.
How does the lifespan of titanium electrodes compare to other materials in saltwater electrolysis?
The lifespan of electrodes is a critical factor in the operational efficiency and cost-effectiveness of saltwater electrolysis systems. When comparing titanium electrodes to other materials commonly used in this application, such as graphite, stainless steel, or platinum-coated substrates, titanium consistently demonstrates superior longevity and performance under the harsh conditions of saltwater electrolysis.
Titanium electrodes typically exhibit an exceptionally long lifespan, often lasting several years or even decades in properly maintained saltwater electrolysis systems. This extended durability is primarily attributed to titanium's inherent corrosion resistance and the formation of a stable, protective oxide layer when exposed to the electrolyte. In contrast, many other electrode materials struggle to withstand the corrosive nature of saltwater and the aggressive conditions of electrolysis for extended periods.
For instance, graphite electrodes, while relatively inexpensive, are prone to erosion and degradation in saltwater environments. The carbon structure of graphite can break down over time, leading to a loss of electrode mass and a decrease in performance. This degradation not only shortens the lifespan of graphite electrodes but can also introduce impurities into the electrolysis process, potentially affecting product quality.
Stainless steel electrodes, despite their corrosion resistance in many applications, can still suffer from pitting and crevice corrosion when used in saltwater electrolysis. The chloride ions present in saltwater can penetrate the passive film of stainless steel, leading to localized corrosion and eventual failure. While some high-grade stainless steels perform better, they still generally fall short of titanium's longevity in these extreme conditions.
Platinum-coated electrodes offer excellent catalytic properties and corrosion resistance but come with significant drawbacks in terms of cost and longevity. The thin platinum coating can wear off over time, exposing the underlying substrate to corrosion. Once the coating is compromised, the electrode's performance rapidly deteriorates, necessitating expensive replacement or recoating.
In comparison, titanium electrodes maintain their structural integrity and performance characteristics over much longer periods. The stable oxide layer that forms on titanium's surface acts as a barrier against corrosion while still allowing for efficient electron transfer. This unique property enables titanium electrodes to withstand the continuous exposure to saltwater and the electrochemical reactions occurring at the electrode surface without significant degradation.

The longevity of titanium electrodes translates into several operational advantages. Firstly, it reduces the frequency of system shutdowns for electrode replacement, minimizing production interruptions and maintenance costs. This aspect is particularly crucial in large-scale industrial applications where downtime can result in substantial financial losses.
Secondly, the consistent performance of titanium electrodes over their extended lifespan ensures stable operation parameters. Unlike electrodes that degrade more rapidly, titanium electrodes maintain their efficiency, allowing for more predictable energy consumption and product output over time. This stability is invaluable for process control and quality assurance in industrial settings.
Moreover, the durability of titanium electrodes contributes to the overall reliability of saltwater electrolysis systems. The reduced risk of sudden electrode failure minimizes the potential for unplanned shutdowns or safety incidents, which is especially important in critical applications such as water treatment or chemical production.
What are the environmental impacts of using titanium electrodes in saltwater electrolysis?
The environmental impact of industrial processes has become an increasingly important consideration in recent years, and saltwater electrolysis is no exception. When evaluating the environmental implications of using titanium electrodes in this application, it's essential to consider various factors, including resource consumption, energy efficiency, waste generation, and the overall life cycle of the electrodes.
One of the primary environmental benefits of using titanium electrodes in saltwater electrolysis is their contribution to improved energy efficiency. The high electrical conductivity of titanium, coupled with its corrosion resistance, allows for efficient electron transfer with minimal energy losses. This efficiency translates into lower power consumption for the electrolysis process, which in turn reduces the carbon footprint associated with electricity generation. In an era where energy conservation and greenhouse gas reduction are critical environmental goals, the energy efficiency of titanium electrodes represents a significant advantage.
The longevity of titanium electrodes also plays a crucial role in their environmental impact. The extended lifespan of these electrodes means that they need to be replaced less frequently compared to other materials. This durability has several positive environmental implications. Firstly, it reduces the demand for raw materials required to manufacture replacement electrodes. The extraction and processing of metals can have substantial environmental impacts, including habitat disruption, energy consumption, and emissions. By minimizing the need for frequent replacements, titanium electrodes help conserve natural resources and reduce the environmental footprint associated with electrode production.
Furthermore, the reduced replacement rate of titanium electrodes leads to less waste generation. When electrodes reach the end of their operational life, they must be disposed of or recycled. Titanium's longevity means that less electrode material enters the waste stream over time, alleviating the burden on waste management systems and reducing the potential for environmental contamination from disposed electrodes.
Titanium itself is a highly recyclable material, which further enhances its environmental credentials. At the end of their service life, titanium electrodes can be recycled and the metal repurposed for other applications. This recyclability helps close the loop in the material's life cycle, reducing the need for virgin titanium production and the associated environmental impacts.
The corrosion resistance of titanium electrodes also contributes to environmental protection in the context of saltwater electrolysis. Unlike some electrode materials that may degrade and release potentially harmful substances into the electrolyte, titanium remains stable under operating conditions. This stability prevents the introduction of unwanted contaminants into the process stream, which is particularly important in applications such as water treatment or the production of chemicals for human consumption.
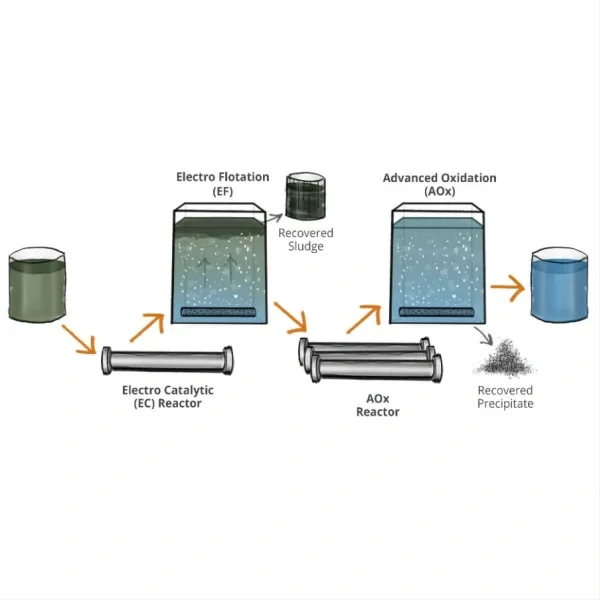
In terms of the electrolysis process itself, titanium electrodes can support more environmentally friendly practices. For instance, in chlor-alkali production, a major industrial application of saltwater electrolysis, titanium electrodes facilitate the use of membrane cell technology. This modern approach is more energy-efficient and environmentally benign compared to older mercury or diaphragm cell processes, as it eliminates the use of mercury and reduces the generation of hazardous waste.
The compatibility of titanium electrodes with various coatings and surface modifications also opens up possibilities for enhancing the environmental performance of electrolysis systems. For example, advanced catalytic coatings can improve the selectivity of the electrolysis process, reducing the formation of unwanted by-products and improving overall efficiency. This selectivity not only enhances product quality but also minimizes waste and reduces the environmental impact of the process.
It's important to note that while titanium electrodes offer many environmental benefits, the production of titanium itself does have environmental considerations. Titanium extraction and processing can be energy-intensive, and the mining of titanium ores can have local environmental impacts. However, when considering the entire life cycle of the electrodes, including their long operational life and recyclability, the net environmental impact is generally favorable compared to alternatives with shorter lifespans.
The use of titanium electrodes also supports the development of emerging green technologies. For instance, in the field of renewable energy, titanium electrodes play a crucial role in water electrolysis for hydrogen production. As the world seeks to transition to cleaner energy sources, the efficiency and durability of titanium electrodes contribute to making hydrogen production more viable and sustainable.
In conclusion, the environmental impacts of using titanium electrodes in saltwater electrolysis are largely positive when considering their entire life cycle. Their energy efficiency, longevity, recyclability, and support for cleaner technologies contribute to reduced resource consumption, lower energy use, and decreased waste generation. While there are environmental considerations in titanium production, the overall benefits of using titanium electrodes in saltwater electrolysis align well with goals for sustainable industrial practices and environmental protection.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References
1. Kraft, A. (2008). Electrochemical Water Disinfection: A Short Review. Platinum Metals Review, 52(3), 177-185.
2. Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
3. Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
4. Chaplin, B. P. (2014). Critical review of electrochemical advanced oxidation processes for water treatment applications. Environmental Science: Processes & Impacts, 16(6), 1182-1203.
5. Sirés, I., Brillas, E., Oturan, M. A., Rodrigo, M. A., & Panizza, M. (2014). Electrochemical advanced oxidation processes: today and tomorrow. A review. Environmental Science and Pollution Research, 21(14), 8336-8367.
6. Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38(1), 11-41.
7. Comninellis, C., & Chen, G. (Eds.). (2010). Electrochemistry for the Environment. Springer Science & Business Media.
8. Rajeshwar, K., & Ibanez, J. G. (1997). Environmental Electrochemistry: Fundamentals and Applications in Pollution Abatement. Academic Press.
9. Pletcher, D., & Walsh, F. C. (1990). Industrial Electrochemistry. Springer Science & Business Media.
10. Bard, A. J., & Faulkner, L. R. (2001). Electrochemical Methods: Fundamentals and Applications. John Wiley & Sons.
Related Industry Knowledge
- How Does Using Electrodeposited Titanium Electrodes Transform Zinc Plating Processes?
- Why Should You Consider Titanium Electrodes for Copper Plating?
- Why is Semiconductor Plating Crucial for Modern Electronics? Understanding the Process and Benefits
- What Industries Rely on DSA Anodes for Electrochemical Processes?
- What Factors Should Be Considered When Selecting a DSA Anode?
- What Is a Chlorine Generator Electrolyzer and How Does It Operate?
- What is a DSA Anode and How Does It Work?
- What Factors Should Be Considered When Selecting a Chlorine Generator Electrolyzer System?