- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
At the forefront of electrochemical advancements lies the development of efficient and durable electrode materials. One such innovation is the DSA (Dimensionally Stable Anode) coating for titanium anodes. These advanced electrodes have revolutionized various industries, from water treatment to electroplating, by offering superior performance and longevity. But what exactly is a DSA coating, and how does it transform the capabilities of titanium anodes?
DSA coatings, first developed in the late 1960s by Henry Beer at Diamond Shamrock Technologies [1], represent a significant leap forward in electrode technology. The coating typically consists of a mixture of precious metals (such as iridium, ruthenium, or platinum) and conductive metal oxides (like titanium dioxide or tantalum oxide) applied to a titanium substrate [2]. This unique composition creates a stable, highly conductive, and catalytically active surface that dramatically enhances the performance of the anode.
The titanium substrate is chosen for its excellent corrosion resistance and relatively low cost compared to other noble metals. However, titanium alone is not an ideal anode material due to its tendency to form a passive oxide layer that reduces conductivity. The DSA coating overcomes this limitation by providing a conductive and catalytically active surface while maintaining the structural integrity and corrosion resistance of the titanium base [3].
How Does DSA Coating Enhance Titanium Anode Performance?
The DSA coating enhances titanium anode performance through several mechanisms:
1. Improved Conductivity: The precious metals and conductive oxides in the coating significantly increase the electrical conductivity of the anode surface. This allows for more efficient electron transfer during electrochemical reactions [4].
2. Catalytic Activity: The mixed metal oxide composition of the coating acts as a catalyst for many electrochemical reactions. This catalytic effect reduces the activation energy required for these reactions, allowing them to proceed at lower potentials and with greater efficiency [5].
3. Corrosion Resistance: While titanium itself is corrosion-resistant, the DSA coating provides an additional layer of protection. The stable oxide structure of the coating resists degradation even in harsh chemical environments, extending the anode's lifespan [6].
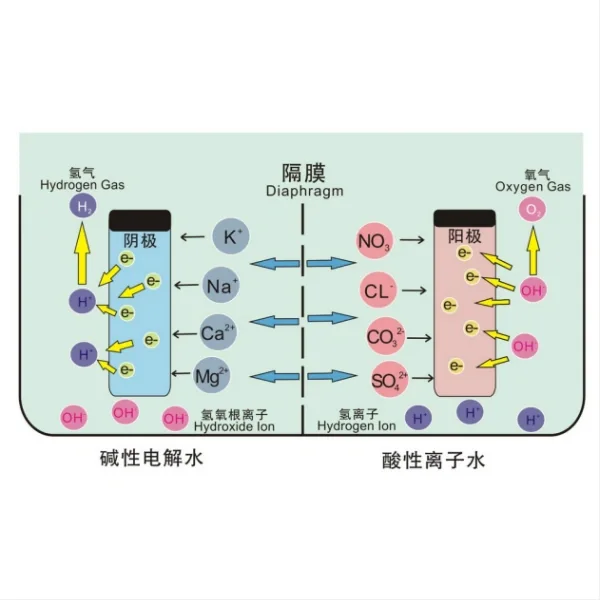
4. Oxygen Evolution Reaction (OER) Efficiency: In processes involving water electrolysis, DSA coatings significantly improve the efficiency of the oxygen evolution reaction. This is particularly important in chlor-alkali production and water treatment applications [7].
5. Dimensional Stability: Unlike traditional anodes that may erode or change shape over time, DSA coated anodes maintain their dimensions throughout their operational life. This stability ensures consistent performance and simplifies system design and maintenance [8].
The enhanced performance of DSA coated titanium anodes is not just a matter of improved efficiency; it also translates to significant economic benefits. The longer lifespan and reduced energy consumption of these anodes often justify their higher initial cost, leading to lower overall operational expenses in many industrial applications [9].
Applications of DSA Coated Titanium Anodes in Electrochemistry: A Deep Dive
The versatility of DSA coated titanium anodes has led to their adoption across a wide range of industries and applications:
1. Chlor-Alkali Industry: In the production of chlorine and sodium hydroxide, DSA anodes have become the standard. They offer high current efficiency, low chlorine overvoltage, and excellent dimensional stability, which are crucial for this energy-intensive process [10].
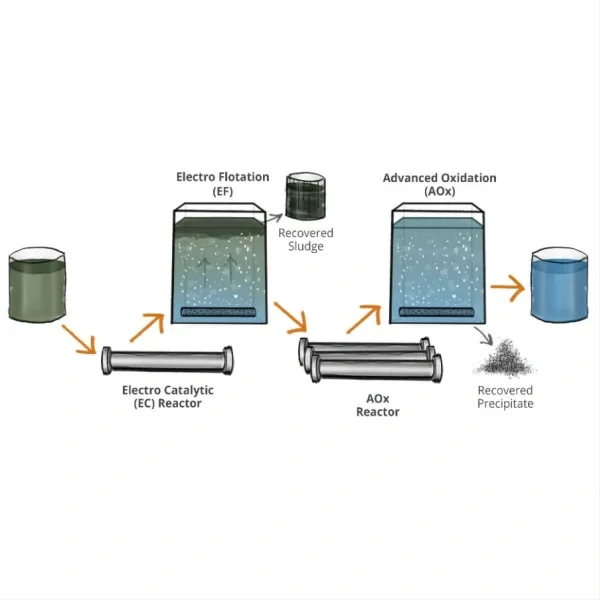
2. Water and Wastewater Treatment: DSA anodes are extensively used in electrochemical advanced oxidation processes (EAOPs) for water purification. They efficiently generate powerful oxidants like hydroxyl radicals and ozone, which can degrade a wide range of pollutants, including recalcitrant organic compounds and pathogens [11].
3. Electroplating and Surface Finishing: The precise control of current distribution offered by DSA Coating Titanium Anodes allows for high-quality metal deposition in electroplating processes. They are particularly useful in hard chrome plating and the production of printed circuit boards [12].

4. Cathodic Protection: DSA anodes are used in impressed current cathodic protection systems to prevent corrosion of metal structures in marine environments, pipelines, and underground storage tanks [13].
5. Electrochemical Synthesis: In the fine chemicals and pharmaceutical industries, DSA anodes facilitate selective organic electrosynthesis reactions, offering a more environmentally friendly alternative to traditional chemical synthesis methods [14].
6. Energy Storage: Research is ongoing into the use of DSA materials in advanced energy storage systems, particularly in redox flow batteries and hydrogen production via water electrolysis [15].
7. Biomedical Applications: DSA coatings are being explored for use in implantable medical devices and biosensors due to their biocompatibility and stability in physiological environments [16].
The wide-ranging applications of DSA coated titanium anodes underscore their importance in modern electrochemistry and industrial processes. As research continues, new applications are likely to emerge, further expanding the utility of these versatile electrodes.
Is DSA Coating the Future of Corrosion-Resistant Anodes?
The future of electrode technology indeed seems to be leaning towards more sustainable and efficient solutions, with DSA coated titanium anodes at the forefront of this trend. Their exceptional corrosion resistance and high performance in electrolysis make them ideal for long-term use in various applications, particularly those involving harsh chemical environments or high current densities.
Current research in DSA technology is focused on several key areas:
1. Novel Coating Compositions: Scientists are exploring new combinations of metals and oxides to create DSA coatings with even higher catalytic activity and stability. For example, the incorporation of rare earth elements like cerium has shown promise in enhancing the performance of DSA Coating Titanium Anodes [17].
2. Nanostructured Coatings: The use of nanotechnology to create DSA coatings with increased surface area and improved catalytic properties is an active area of research. Nanostructured coatings can potentially offer higher efficiency and lower overpotentials [18].
3. Sustainable Production Methods: Efforts are being made to develop more environmentally friendly methods for producing DSA coatings, including the use of less toxic precursors and more energy-efficient deposition techniques [19].
4. Advanced Characterization Techniques: The development of new methods to analyze the structure and performance of DSA coatings at the atomic level is crucial for optimizing their design and performance [20].
5. Multifunctional Coatings: Research is ongoing into DSA coatings that can perform multiple functions, such as simultaneous pollutant degradation and disinfection in water treatment applications [21].
While DSA coated titanium anodes have already proven their worth in many applications, ongoing research and development promise to further expand their capabilities and efficiency. As industries continue to prioritize sustainability and energy efficiency, the demand for these advanced electrodes is likely to grow.
Conclusion
The DSA Coating Titanium Anodes represents a significant leap forward in electrode technology. By offering enhanced performance, corrosion resistance, and efficiency, these anodes are set to play a crucial role in the advancement of electrochemistry. As industries continue to adopt cleaner and more sustainable practices, the DSA coated titanium anode stands out as a promising solution for a wide range of applications.

The continued research and development in this field suggest that we have only scratched the surface of what DSA technology can offer. From more efficient industrial processes to advanced energy storage solutions and novel medical applications, the future of DSA coated titanium anodes looks bright. As we face growing environmental challenges and the need for more sustainable technologies, these versatile electrodes are likely to play an increasingly important role in shaping the future of electrochemistry and industrial processes.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
[1] Beer, H. B. (1980). The invention and industrial development of metal anodes. Journal of the Electrochemical Society, 127(8), 303C.
[2] Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
[3] Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
[4] Comninellis, C., & Chen, G. (Eds.). (2010). Electrochemistry for the Environment. Springer Science & Business Media.
[5] Špalek, O., Balej, J., & Paseka, I. (1987). Hydrogen evolution on titanium electrodes with platinum and iridium surface coatings. Journal of Applied Electrochemistry, 17(5), 1113-1115.
[6] Aromaa, J., & Forsén, O. (2006). Evaluation of the electrochemical activity of a Ti–RuO2–TiO2 permanent anode. Electrochimica Acta, 51(27), 6104-6110.
[7] Trasatti, S. (1984). Electrocatalysis in the anodic evolution of oxygen and chlorine. Electrochimica Acta, 29(11), 1503-1512.
[8] Kraft, A. (2007). Doped diamond: a compact review on a new, versatile electrode material. International Journal of Electrochemical Science, 2, 355-385.
[9] Chaplin, B. P. (2014). Critical review of electrochemical advanced oxidation processes for water treatment applications. Environmental Science: Processes & Impacts, 16(6), 1182-1203.
[10] O'Brien, T. F., Bommaraju, T. V., & Hine, F. (2005). Handbook of chlor-alkali technology. Springer Science & Business Media.
[11] Martínez-Huitle, C. A., & Brillas, E. (2009). Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Applied Catalysis B: Environmental, 87(3-4), 105-145.
[12] Walsh, F. C., & Ponce de León, C. (2014). A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying technology. Transactions of the IMF, 92(2), 83-98.
[13] Shabani-Nooshabadi, M., & Ghoreishi, S. M. (2015). Electrochemical reduction of perfluorooctanoic acid using various electrode materials: mechanism, kinetics, and influence of supporting electrolyte. Journal of Electroanalytical Chemistry, 747, 120-129.
[14] Frontana-Uribe, B. A., Little, R. D., Ibanez, J. G., Palma, A., & Vasquez-Medrano, R. (2010). Organic electrosynthesis: a promising green methodology in organic chemistry. Green Chemistry, 12(12), 2099-2119.
[15] Wang, W., Luo, Q., Li, B., Wei, X., Li, L., & Yang, Z. (2013). Recent progress in redox flow battery research and development. Advanced Functional Materials, 23(8), 970-986.
[16] Cogan, S. F. (2008). Neural stimulation and recording electrodes. Annual Review of Biomedical Engineering, 10, 275-309.
[17] Chen, R., Trieu, V., Zeradjanin, A. R., Natter, H., Teschner, D., Kintrup, J., ... & Schuhmann, W. (2012). Microstructural impact of anodic coatings on the electrochemical chlorine evolution reaction. Physical Chemistry Chemical Physics, 14(20), 7392-7399.
[18] Xu, L., Xiao, Y., van Sandwijk, A., Xu, Q., & Yang, Y. (2015). Production of nanostructured TiO2 by controlling hydrolysis in sol–gel process. Materials Research Bulletin, 61, 19-24.
[19] Morais, A., Alves, J. C., Lima, F. A. S., Lira-Cantu, M., & Nogueira, A. F. (2015). Enhanced photovoltaic performance of inverted hybrid bulk-heterojunction solar cells using TiO2/reduced graphene oxide films as electron transport layers. Journal of Photonics for Energy, 5(1), 057408.
[20] Binninger, T., Mohamed, R., Waltar, K., Fabbri, E., Levecque, P., Kötz, R., & Schmidt, T. J. (2015). Thermodynamic explanation of the universal correlation between oxygen evolution activity and corrosion of oxide catalysts. Scientific Reports, 5(1), 1-7.
[21] Martínez-Huitle, C. A., Rodrigo, M. A., Sirés, I., & Scialdone, O. (2015). Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chemical Reviews, 115(24), 13362-13407.
Related Industry Knowledge
- What Benefits Does DSA Coating Provide For Titanium Anodes?
- How Does the DSA Coating on Titanium Anodes Work?
- What is the Lifespan of a DSA Coated Titanium Anode?
- How Does a DSA Anode Revolutionize Electrochemical Processes?
- What are DSA Anodes?
- What Industries Rely on DSA Anodes for Electrochemical Processes?
- What Factors Should Be Considered When Selecting a DSA Anode?
- What is a DSA Anode and How Does It Work?
- What are DSA anodes?
- What are the Typical Applications of DSA Coating Titanium Anodes?