- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Dimensionally Stable Anodes (DSA) with titanium substrates have revolutionized electrochemical processes across various industries. The DSA coating on titanium anodes is a sophisticated electrochemical innovation that enhances the performance and longevity of these critical components. This coating typically consists of a mixture of noble metal oxides, such as ruthenium, iridium, or platinum, applied to a titanium base. The unique properties of DSA coatings allow for stable, efficient, and long-lasting electrochemical reactions, making them indispensable in applications ranging from chlorine production to water treatment.
What are the advantages of using DSA coated titanium anodes?
The adoption of DSA coated titanium anodes has brought about significant improvements in electrochemical processes, offering a multitude of advantages over traditional anode materials. These benefits have made DSA coated titanium anodes the preferred choice in many industrial applications.
One of the primary advantages of DSA coated titanium anodes is their exceptional durability and longevity. The coating's composition, typically a mixture of noble metal oxides, provides remarkable resistance to corrosion and erosion, even in highly aggressive electrolytic environments. This resilience translates to extended operational lifetimes, often measuring in years or even decades, depending on the specific application and operating conditions. The longevity of these anodes not only reduces the frequency of replacements but also minimizes production downtime, leading to significant cost savings for industries relying on electrochemical processes.
Another crucial advantage is the high electrocatalytic activity of DSA coatings. The carefully engineered composition of these coatings, often including oxides of ruthenium, iridium, or platinum, results in a surface with exceptional catalytic properties. This enhanced catalytic activity allows for more efficient electron transfer reactions, reducing the overpotential required for desired electrochemical reactions. As a result, DSA coated titanium anodes can operate at lower voltages compared to traditional anodes, leading to substantial energy savings in large-scale industrial processes.
The dimensional stability of DSA coated anodes is another significant benefit, as implied by their name. Unlike some traditional anode materials that may undergo substantial dimensional changes during operation due to wear or chemical reactions, DSA coated titanium anodes maintain their shape and size over extended periods. This dimensional stability ensures consistent performance and uniform current distribution across the anode surface, which is critical for maintaining product quality and process efficiency in applications such as electroplating or chlorine production.
Furthermore, DSA coated titanium anodes offer excellent selectivity for desired reactions. The composition of the coating can be tailored to promote specific electrochemical reactions while suppressing unwanted side reactions. This selectivity is particularly valuable in processes where product purity is paramount, such as in the production of high-purity chemicals or in advanced water treatment systems.
The versatility of DSA coated titanium anodes is yet another advantage that contributes to their widespread adoption. These anodes can be designed and manufactured in various shapes and sizes to suit specific reactor configurations and process requirements. Whether it's flat plates for membrane electrolyzers, cylindrical forms for tube-type electrolytic cells, or complex geometries for specialized applications, DSA coated titanium anodes can be customized to optimize performance in diverse electrochemical systems.
Lastly, the use of DSA coated titanium anodes often results in improved product quality and process consistency. The stable performance and uniform current distribution provided by these anodes lead to more predictable and controllable electrochemical reactions. This consistency is crucial in industries where product specifications are stringent, such as in the production of electronic-grade chemicals or in precise metal recovery processes.
How is the DSA coating applied to titanium anodes?
The application of DSA coating to titanium anodes is a sophisticated process that requires precision and careful control to achieve the desired electrochemical properties. The coating procedure typically involves several steps, each critical to ensuring the final product's performance and durability.
The process begins with the preparation of the titanium substrate. The titanium surface must be meticulously cleaned and often roughened to promote better adhesion of the coating. This preparation may involve mechanical abrasion, chemical etching, or a combination of both. The goal is to create a clean, oxide-free surface with increased surface area, which will enhance the bonding between the coating and the substrate.
Once the titanium surface is prepared, the application of the DSA coating commences. There are several methods for applying the coating, with thermal decomposition being one of the most common. In this method, a precursor solution containing the desired metal salts (typically chlorides of ruthenium, iridium, or other noble metals) is applied to the titanium surface. This can be done through various techniques such as brushing, dipping, or spraying, depending on the geometry of the anode and the desired coating thickness.
After the precursor solution is applied, the coated titanium undergoes a thermal treatment process. This involves heating the anode to high temperatures, typically between 400°C and 500°C, in a controlled atmosphere. During this heating process, the precursor compounds decompose, forming a layer of metal oxides that strongly adhere to the titanium surface. The heating process is often repeated multiple times, with fresh precursor solution applied between each heating cycle. This layering approach helps to build up the desired coating thickness and composition.
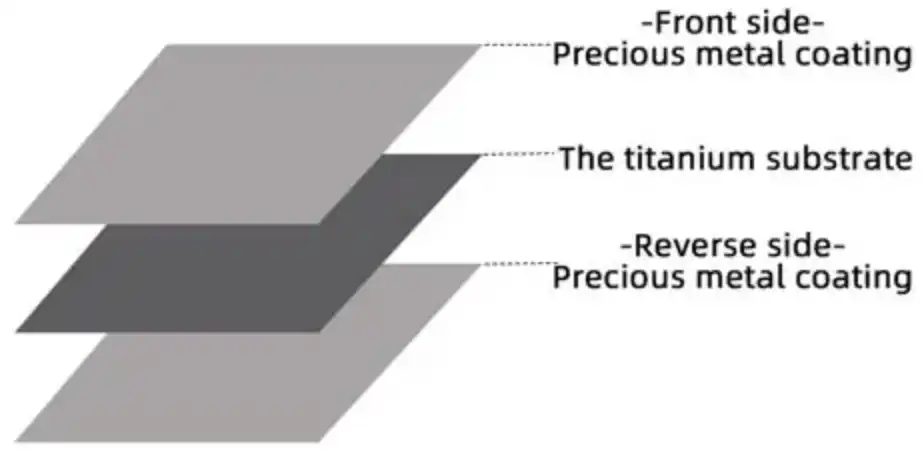
The thermal decomposition process is critical in determining the final properties of the DSA coating. The temperature, duration, and atmosphere of the heating cycles all play roles in the formation of the oxide structure. These parameters can be adjusted to optimize the coating's catalytic activity, stability, and conductivity for specific applications.
An alternative method for applying DSA coatings is electrodeposition. In this technique, the titanium substrate is immersed in an electrolyte solution containing the desired metal ions. An electric current is passed through the solution, causing the metal ions to deposit onto the titanium surface. This method can offer more precise control over the coating composition and thickness but may require additional post-deposition treatments to achieve the desired oxide structure.
Advanced coating techniques may also incorporate the use of intermediate layers or dopants to enhance specific properties of the DSA coating. For example, a thin layer of titanium oxide might be intentionally formed on the substrate before the main coating application to improve adhesion. Similarly, small amounts of additional elements might be introduced into the coating to modify its catalytic or conductive properties.
The final step in the coating process often involves a post-treatment phase. This may include additional heat treatment to further stabilize the oxide structure or surface treatments to activate the catalytic properties of the coating. Some manufacturers may also apply protective outer layers to enhance the anode's resistance to specific chemical environments.
Throughout the coating process, strict quality control measures are essential. The thickness, composition, and structure of the coating are carefully monitored using various analytical techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), and electrochemical testing. These analyses ensure that the coating meets the required specifications for its intended application.
It's worth noting that the exact details of DSA coating processes are often closely guarded trade secrets, with manufacturers continually refining their techniques to improve performance and reduce costs. The ability to consistently produce high-quality DSA coatings on titanium anodes requires not only sophisticated equipment but also considerable expertise and experience.
What applications benefit from DSA coated titanium anodes?
DSA coated titanium anodes have found widespread use across numerous industries due to their exceptional performance characteristics. Their unique combination of durability, efficiency, and versatility has made them indispensable in a wide range of electrochemical applications.

One of the most significant applications of DSA coated titanium anodes is in the chlor-alkali industry. This industry, which produces chlorine, sodium hydroxide, and hydrogen through the electrolysis of brine, has been revolutionized by the introduction of DSA technology. The high catalytic activity of DSA coatings, particularly those based on ruthenium and iridium oxides, allows for efficient chlorine evolution at the anode. The dimensional stability of these anodes ensures consistent performance over long periods, which is crucial in the continuous operation of chlor-alkali plants. Moreover, the resistance of DSA coatings to the highly corrosive chlorine environment significantly extends the lifespan of the anodes, reducing maintenance costs and improving overall process economics.
Water and wastewater treatment represent another major area where DSA coated titanium anodes have made a substantial impact. In electrochemical water treatment processes, these anodes are used for the generation of powerful oxidants such as ozone, hydrogen peroxide, and hydroxyl radicals. These oxidants are effective in breaking down organic pollutants, destroying pathogens, and removing color and odor from water. The ability of DSA coatings to promote these reactions efficiently, even at low concentrations of pollutants, makes them particularly valuable in advanced oxidation processes for treating recalcitrant contaminants. Additionally, DSA coated anodes are employed in electrocoagulation systems for removing heavy metals and suspended solids from industrial wastewater.

The metal finishing and electroplating industries also benefit significantly from DSA coated titanium anodes. In these applications, the anodes are used in processes such as electrowinning, where metals are extracted from their ores or recovered from solutions through electrodeposition. The stability and longevity of DSA coated anodes in acidic electrolytes make them ideal for these processes, where consistent performance is crucial for product quality. Moreover, the ability to tailor the coating composition allows for optimization of the anode's performance for specific metal recovery processes.
In the field of cathodic protection, DSA coated titanium anodes play a vital role in protecting metal structures from corrosion. These anodes are used in impressed current cathodic protection (ICCP) systems to protect pipelines, storage tanks, marine structures, and reinforced concrete from corrosive environments. The long lifespan and stable performance of DSA coated anodes ensure reliable protection over extended periods, even in harsh environments such as seawater or corrosive soils.
The electronics industry utilizes DSA coated titanium anodes in the production of printed circuit boards (PCBs) and in electroforming processes. The precise control of current distribution made possible by these anodes is crucial for achieving uniform metal deposition and etching in PCB manufacturing. In electroforming, where metal parts are created through electrodeposition on a mandrel, the stability and efficiency of DSA coated anodes contribute to the production of high-quality, dimensionally accurate components.
Emerging applications for DSA coated titanium anodes include their use in advanced energy storage and conversion technologies. In flow batteries, for example, these anodes are being explored for their potential to enhance the efficiency and lifespan of large-scale energy storage systems. Similarly, in water electrolysis for hydrogen production, DSA coated anodes are being investigated for their ability to improve the efficiency of oxygen evolution reactions, a critical step in green hydrogen production.
The pharmaceutical and fine chemicals industries also benefit from DSA coated titanium anodes in electroorganic synthesis processes. These anodes facilitate selective oxidation reactions, enabling the production of complex organic compounds through environmentally friendly electrochemical routes, as opposed to traditional chemical synthesis methods that may require harsh reagents or generate significant waste.
In conclusion, the versatility and superior performance of DSA coated titanium anodes have led to their adoption across a diverse range of industries and applications. From large-scale chemical production to environmental remediation and emerging energy technologies, these anodes continue to play a crucial role in advancing electrochemical processes, improving efficiency, and enabling new technological possibilities.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References
1. Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
2. Karlsson, R. K., & Cornell, A. (2016). Selectivity between oxygen and chlorine evolution in the chlor-alkali and chlorate processes. Chemical Reviews, 116(5), 2982-3028.
3. Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38(1), 11-41.
4. Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
5. Kraft, A. (2007). Doped diamond: a compact review on a new, versatile electrode material. International Journal of Electrochemical Science, 2(5), 355-385.
6. Comninellis, C., & Chen, G. (Eds.). (2010). Electrochemistry for the Environment. Springer Science & Business Media.
7. Panizza, M., & Cerisola, G. (2009). Direct and mediated anodic oxidation of organic pollutants. Chemical Reviews, 109(12), 6541-6569.
8. Kotz, R., Stucki, S., & Carcer, B. (1991). Electrochemical waste water treatment using high overvoltage anodes. Part I: Physical and electrochemical properties of SnO2 anodes. Journal of Applied Electrochemistry, 21(1), 14-20.
9. Rajeshwar, K., & Ibanez, J. G. (1997). Environmental electrochemistry: Fundamentals and applications in pollution sensors and abatement. Academic Press.
10. Simond, O., Schaller, V., & Comninellis, C. (1997). Theoretical model for the anodic oxidation of organics on metal oxide electrodes. Electrochimica Acta, 42(13-14), 2009-2012.
Related Industry Knowledge
- What Benefits Does DSA Coating Provide For Titanium Anodes?
- What is the Lifespan of a DSA Coated Titanium Anode?
- How Does a DSA Anode Revolutionize Electrochemical Processes?
- What is a DSA Coating Titanium Anode?
- What are DSA Anodes?
- What Industries Rely on DSA Anodes for Electrochemical Processes?
- What Factors Should Be Considered When Selecting a DSA Anode?
- What is a DSA Anode and How Does It Work?
- What are DSA anodes?
- What are the Typical Applications of DSA Coating Titanium Anodes?












