- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
How to Choose the Right Titanium Electrode for Your Seawater Electrolysis Project?
Selecting the appropriate titanium electrode is crucial for the success of any seawater electrolysis project. Titanium electrodes are widely used in this field due to their excellent corrosion resistance and durability in harsh marine environments. However, choosing the right electrode involves considering various factors such as the specific application, desired performance, and environmental conditions. This blog post will guide you through the key considerations and provide insights to help you make an informed decision for your seawater electrolysis project.
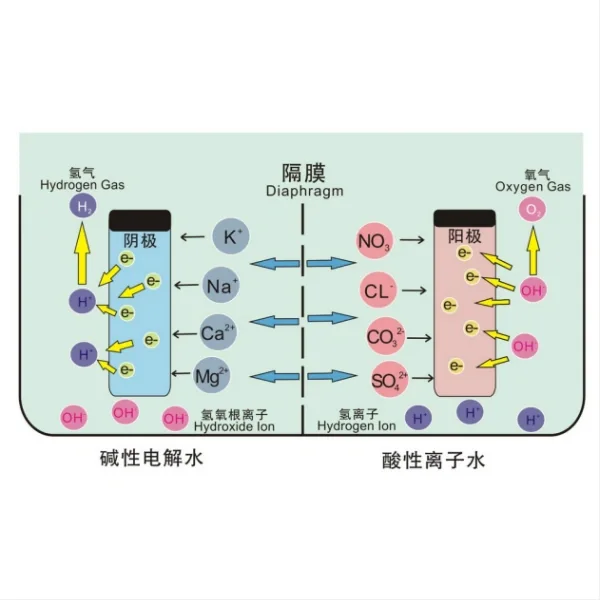
What are the benefits of using titanium electrodes in seawater electrolysis?
Titanium electrodes offer numerous advantages in seawater electrolysis applications, making them a popular choice among researchers and industry professionals. The primary benefits of using titanium electrodes include:
1. Exceptional corrosion resistance: Titanium forms a stable oxide layer when exposed to oxygen, providing excellent protection against corrosion in aggressive marine environments. This property ensures the longevity of the electrode, reducing maintenance costs and downtime.
2. High strength-to-weight ratio: Titanium electrodes are lightweight yet strong, making them ideal for large-scale installations where weight is a concern. This characteristic also facilitates easier handling and installation.
3. Excellent conductivity: While pure titanium is not as conductive as some other metals, it can be coated or alloyed to enhance its electrical conductivity. This makes it suitable for efficient electron transfer in electrolysis processes.
4. Versatility: Titanium electrodes can be manufactured in various shapes and sizes, allowing for customization based on specific project requirements. They can also be coated with different materials to optimize their performance for particular applications.
5. Long lifespan: Due to their corrosion resistance and durability, titanium electrodes have a longer operational life compared to many other electrode materials. This results in reduced replacement frequency and lower long-term costs.
6. Biocompatibility: In applications where biological considerations are important, titanium's biocompatibility is an added advantage. This property makes it suitable for use in environmentally sensitive areas or in projects where biofouling is a concern.
These benefits collectively make titanium electrodes an excellent choice for seawater electrolysis projects. However, it's important to note that the specific advantages may vary depending on the particular application, electrode coating, and operating conditions. Careful consideration of these factors is essential when selecting the most appropriate titanium electrode for your project.
How does electrode coating affect the performance of titanium electrodes in seawater electrolysis?
The coating applied to titanium electrodes plays a crucial role in determining their performance in seawater electrolysis. While titanium itself is an excellent base material due to its corrosion resistance, the coating significantly enhances its electrochemical properties and overall efficiency. Understanding the impact of electrode coatings is essential for optimizing your seawater electrolysis process:
1. Catalytic activity: Coatings can dramatically increase the catalytic activity of titanium electrodes. Common coating materials include platinum group metals (PGMs) such as ruthenium, iridium, and platinum, as well as mixed metal oxides (MMOs). These coatings lower the overpotential required for the electrolysis reactions, improving energy efficiency.
2. Selectivity: Different coatings can promote selectivity towards specific reactions. For instance, certain coatings may favor oxygen evolution over chlorine evolution, which can be crucial depending on the desired products of your electrolysis process.
3. Conductivity enhancement: While titanium forms a passive oxide layer that protects against corrosion, this layer can also increase electrical resistance. Conductive coatings help overcome this limitation, improving the overall conductivity of the electrode.
4. Stability in seawater: Some coatings provide additional protection against the corrosive effects of seawater, extending the lifespan of the electrode beyond what bare titanium can offer.
5. Resistance to fouling: Certain coatings can impart anti-fouling properties, reducing the build-up of organic and inorganic deposits on the electrode surface. This helps maintain performance over time and reduces maintenance requirements.
6. Current density: Coatings can allow for higher current densities to be applied without damaging the electrode, potentially increasing the productivity of your electrolysis system.
When selecting a coated titanium electrode for your seawater electrolysis project, consider the following factors:
- Energy efficiency: PGM coatings generally offer higher efficiency but come at a higher cost. Evaluate the trade-off between initial investment and long-term energy savings.
- Operating conditions: Consider factors such as temperature, pH, and expected current densities when choosing a coating that will provide optimal performance and longevity under your specific conditions.
- Maintenance requirements: Some coatings are more resistant to fouling and degradation, potentially reducing the frequency of electrode replacement or cleaning.
- Cost considerations: While PGM coatings offer excellent performance, they can be expensive. MMO coatings might provide a more cost-effective solution for some applications.
It's important to work closely with electrode manufacturers or consult with experts in the field to determine the most appropriate coating for your specific seawater electrolysis project. They can provide guidance based on the latest developments in coating technology and help you balance performance requirements with budget constraints.
Remember that the effectiveness of a coated titanium electrode also depends on proper installation, operation within specified parameters, and regular maintenance. By carefully considering the coating options and their impacts on performance, you can optimize your seawater electrolysis process for efficiency, longevity, and desired outcomes.
What factors should be considered when sizing titanium electrodes for a seawater electrolysis system?
Proper sizing of titanium electrodes is critical for the efficient and effective operation of a seawater electrolysis system. The size and configuration of the electrodes directly impact the system's performance, energy consumption, and overall cost-effectiveness. Here are the key factors to consider when sizing titanium electrodes for your seawater electrolysis project:
1. Current density requirements: The current density (current per unit area of electrode surface) is a fundamental parameter in electrode sizing. It affects reaction rates, energy efficiency, and electrode lifespan. Determine the optimal current density for your specific application, considering that:
- Higher current densities can increase production rates but may lead to faster electrode degradation and higher energy consumption.
- Lower current densities may be more energy-efficient and gentler on the electrodes but result in lower production rates.
2. Total current and production capacity: Calculate the total current needed to achieve your desired production capacity. This, combined with the optimal current density, will help determine the total electrode surface area required.
3. Electrode geometry: Consider the shape and dimensions of the electrodes. Factors to consider include:
- Plate vs. mesh designs: Mesh electrodes offer higher surface area but may be more prone to clogging in some applications.
- Thickness: Thicker electrodes last longer but increase material costs and may affect current distribution.
- Surface-to-volume ratio: Higher ratios generally improve mass transfer and reaction efficiency.
4. Inter-electrode gap: The distance between anodes and cathodes affects energy efficiency and product quality. A smaller gap typically improves efficiency but may increase the risk of short circuits or uneven current distribution.
5. Flow dynamics: Consider how the seawater will flow through your electrolysis cell. Electrode sizing and arrangement should promote uniform flow distribution to ensure consistent reaction conditions across the electrode surface.
6. Scale-up considerations: If you're designing a large-scale system, consider how electrode sizing will affect modularity and scalability. Larger electrodes may offer economies of scale but can be more challenging to manufacture, transport, and install.
7. Operating parameters: Factor in the expected operating conditions, including:
- Temperature: Higher temperatures may allow for higher current densities but can also accelerate electrode degradation.
- Salinity: Variations in seawater salinity can affect conductivity and thus impact electrode performance.
- pH: Extreme pH conditions may require more robust electrode designs or coatings.
8. Pressure drop: In flow-through systems, electrode size and configuration affect the pressure drop across the cell. Balance the need for sufficient electrode area with the energy costs associated with pumping.
9. Maintenance and replacement: Consider how electrode size will impact maintenance procedures and replacement frequency. Larger electrodes may be more challenging to handle but could reduce the frequency of replacements.
10. Cost optimization: Balance the initial costs of larger or more numerous electrodes against potential long-term savings in energy and maintenance. Sometimes, a higher upfront investment in electrode capacity can lead to significant operational cost reductions.
11. Space constraints: If your system has spatial limitations, electrode sizing must be optimized to fit within the available space while meeting performance requirements.
12. Uniformity of current distribution: Proper sizing and arrangement of electrodes help ensure uniform current distribution, which is crucial for consistent product quality and electrode longevity.
To effectively size titanium electrodes for your seawater electrolysis system, follow these steps:
1. Define your production goals and system requirements clearly.
2. Consult with electrode manufacturers or electrochemical engineers to determine the optimal current density for your specific application.
3. Calculate the total electrode surface area needed based on your production goals and optimal current density.
4. Consider the practical aspects of electrode design, including geometry, inter-electrode gap, and flow dynamics.
5. Evaluate different electrode configurations using computational modeling or small-scale experiments if possible.
6. Factor in scalability, maintenance, and long-term operational costs.
7. Iterate on your design, balancing performance, cost, and practical considerations.
Remember that electrode sizing is often an iterative process, and it may be beneficial to test different configurations on a smaller scale before committing to a full-scale design. Additionally, as technology in this field is continually evolving, stay informed about the latest developments in titanium electrode design and coating technologies that may impact sizing considerations.
By carefully considering these factors and following a systematic approach to electrode sizing, you can optimize your seawater electrolysis system for efficiency, longevity, and cost-effectiveness, ensuring the success of your project.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References
1. Kraft, A. (2008). Electrochemical water disinfection: A short review. Platinum Metals Review, 52(3), 177-185.
2. Rajeshwar, K., & Ibanez, J. G. (1997). Environmental electrochemistry: Fundamentals and applications in pollution abatement. Academic Press.
3. Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
4. Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
5. Comninellis, C., & Chen, G. (Eds.). (2010). Electrochemistry for the Environment. Springer Science & Business Media.
6. Pillai, K. C., Kwon, T. O., & Moon, I. S. (2009). Degradation of wastewater from terephthalic acid manufacturing process by ozonation catalyzed with Fe2+, H2O2 and UV light: Direct versus indirect ozonation reactions. Applied Catalysis B: Environmental, 91(1-2), 319-328.
7. Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38(1), 11-41.
8. Panizza, M., & Cerisola, G. (2009). Direct and mediated anodic oxidation of organic pollutants. Chemical Reviews, 109(12), 6541-6569.
9. Chaplin, B. P. (2014). Critical review of electrochemical advanced oxidation processes for water treatment applications. Environmental Science: Processes & Impacts, 16(6), 1182-1203.
10. Sirés, I., Brillas, E., Oturan, M. A., Rodrigo, M. A., & Panizza, M. (2014). Electrochemical advanced oxidation processes: today and tomorrow. A review. Environmental Science and Pollution Research, 21(14), 8336-8367.
Related Industry Knowledge
- How Does a Chlorine Electrolysis Cell Operate?
- What are the Benefits of Using Titanium Electrodes in the Electrodeposition Process for Nickel And Cobalt?
- Why is Titanium Mesh Used?
- Can Titanium Electrodes be Used for Large-Scale Water Treatment?
- How Does a DSA Anode Revolutionize Electrochemical Processes?
- What Is an MMO Anode Plate and How Does It Function in Electrochemical Processes?
- What Industries Rely on DSA Anodes for Electrochemical Processes?