- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
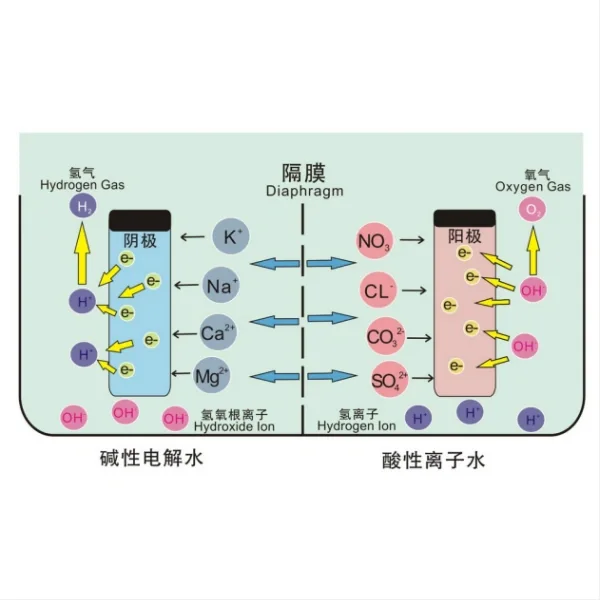
Titanium mesh is a versatile material that has gained significant popularity in various industries due to its unique properties. This lightweight yet strong material offers excellent corrosion resistance, biocompatibility, and durability, making it an ideal choice for numerous applications. In this blog post, we'll explore the reasons behind the widespread use of titanium mesh, focusing particularly on its role in electrode-diaphragm assemblies for alkaline water electrolysis.
How does titanium mesh improve electrode performance in alkaline water electrolysis?
Titanium mesh plays a crucial role in enhancing electrode performance in alkaline water electrolysis. This advanced material offers several benefits that contribute to the overall efficiency and effectiveness of the electrolysis process.
Firstly, titanium mesh provides an excellent substrate for catalytic coatings. The mesh structure offers a high surface area, allowing for a greater distribution of catalytic materials such as platinum, iridium, or nickel. This increased surface area results in more active sites for the electrochemical reactions to occur, leading to improved electrode performance.
The corrosion resistance of titanium is another key factor in its effectiveness as an electrode material. In the highly alkaline environment of water electrolysis, many materials would quickly degrade. However, titanium forms a stable oxide layer on its surface, protecting it from corrosion and ensuring long-term durability. This stability is crucial for maintaining consistent electrode performance over extended periods of operation.
Furthermore, the electrical conductivity of titanium mesh contributes to its effectiveness as an electrode material. While not as conductive as some other metals, titanium offers a good balance between conductivity and corrosion resistance. The mesh structure also allows for efficient electron transfer throughout the electrode, facilitating the electrochemical reactions.
The porous nature of titanium mesh also aids in gas evolution during electrolysis. As hydrogen and oxygen are produced at the cathode and anode respectively, the mesh structure allows these gases to escape easily, preventing gas buildup that could impede the reaction. This efficient gas release mechanism helps maintain a stable and continuous electrolysis process.
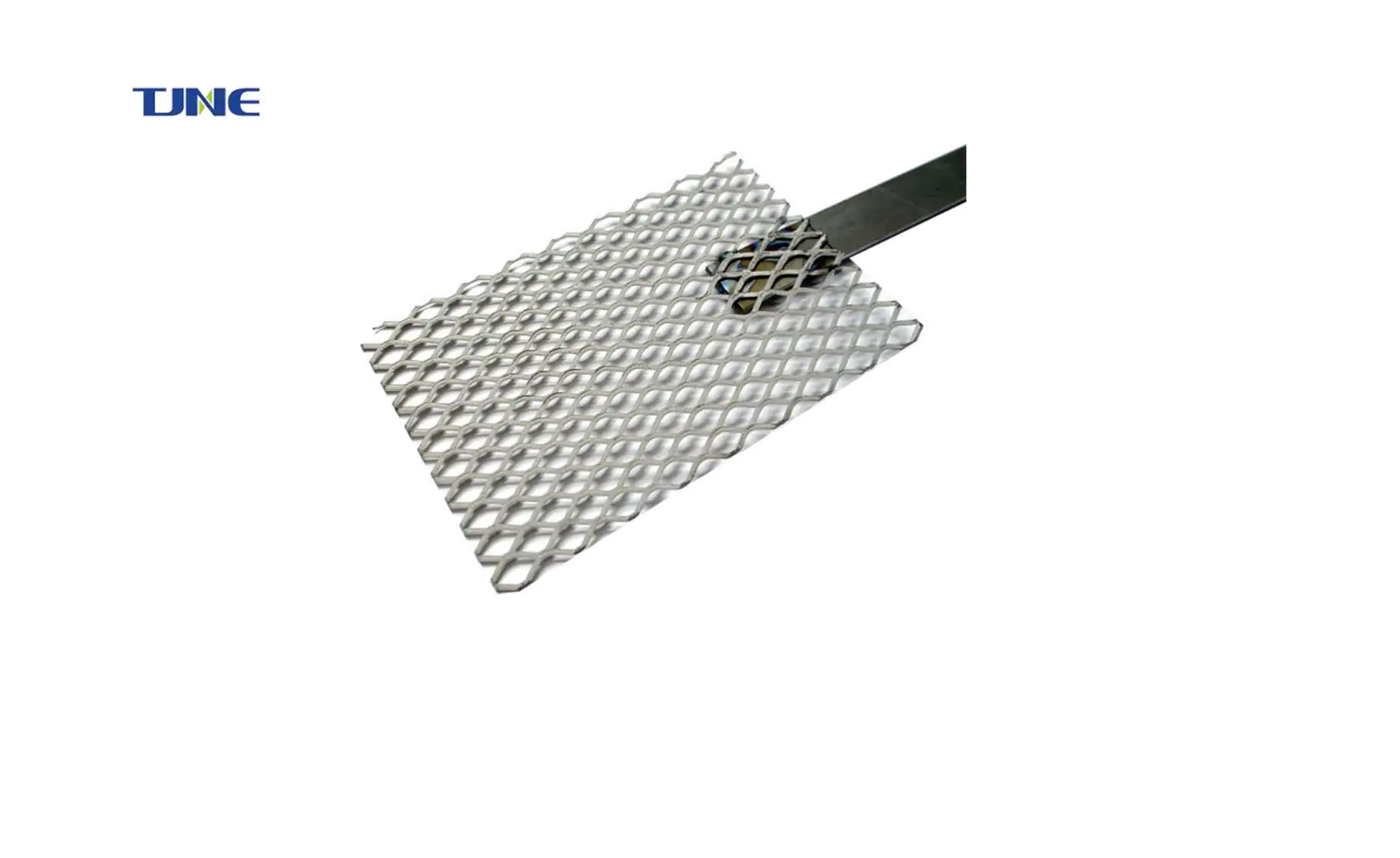
Additionally, titanium mesh electrodes can be easily customized to suit specific electrolysis requirements. The mesh size, thickness, and overall geometry can be tailored to optimize performance for different electrolysis systems. This flexibility allows for fine-tuning of electrode designs to achieve maximum efficiency in various alkaline water electrolysis applications.
In terms of longevity, titanium mesh electrodes significantly outperform many alternatives. The combination of corrosion resistance and mechanical strength means that these electrodes can withstand the harsh conditions of alkaline electrolysis for extended periods. This longevity translates to reduced maintenance requirements and longer operational lifetimes for electrolysis systems, contributing to overall cost-effectiveness.
What are the advantages of using titanium mesh in electrode-diaphragm assemblies?
The use of titanium mesh in electrode-diaphragm assemblies for alkaline water electrolysis offers numerous advantages that contribute to the overall performance and efficiency of the system.
One of the primary advantages is the mechanical support provided by the titanium mesh. In electrode-diaphragm assemblies, the mesh acts as a robust framework that supports the catalytic coating and the diaphragm material. This structural integrity is crucial for maintaining the proper configuration of the assembly during the electrolysis process, ensuring consistent performance and preventing physical degradation of the components.
The open structure of titanium mesh also facilitates excellent mass transport properties. In alkaline water electrolysis, efficient transport of reactants to the electrode surface and removal of products is essential for maintaining high reaction rates. The mesh structure allows for easy passage of electrolyte solutions, ensuring that fresh reactants constantly reach the active sites on the electrode surface. Similarly, it aids in the efficient removal of gaseous products, preventing gas accumulation that could hinder the electrolysis process.
Another significant advantage is the compatibility of titanium mesh with various diaphragm materials. The mesh can be effectively combined with polymer-based or ceramic diaphragms, providing a stable interface between the electrode and the separator. This compatibility ensures good adhesion and prevents delamination issues that could compromise the performance of the electrode-diaphragm assembly.
The use of titanium mesh also contributes to uniform current distribution across the electrode surface. The mesh structure helps to spread the electric current evenly, reducing the likelihood of localized high-current density areas that could lead to hotspots or accelerated degradation of the electrode material. This uniform current distribution is crucial for maintaining consistent electrolysis performance and extending the operational lifetime of the electrode assembly.
Furthermore, titanium mesh electrode-diaphragm assemblies offer excellent scalability. The mesh structure can be easily scaled up or down to suit different electrolysis cell sizes without compromising performance. This scalability is particularly advantageous for industrial applications where large-scale hydrogen production is required.
The thermal management capabilities of titanium mesh are another noteworthy advantage. During the electrolysis process, heat is generated as a byproduct of the electrochemical reactions. The open structure of the mesh allows for effective heat dissipation, helping to maintain optimal operating temperatures within the electrolysis cell. This thermal management is crucial for preventing overheating issues that could degrade the electrode materials or affect the overall system efficiency.
Lastly, the use of titanium mesh in electrode-diaphragm assemblies contributes to the overall robustness and reliability of alkaline water electrolysis systems. The combination of corrosion resistance, mechanical strength, and stable performance characteristics makes these assemblies well-suited for long-term operation in industrial settings. This reliability translates to reduced downtime for maintenance or replacement, leading to improved operational efficiency and cost-effectiveness in hydrogen production facilities.
Can titanium mesh enhance the efficiency of hydrogen production in water electrolysis?
The use of titanium mesh can indeed enhance the efficiency of hydrogen production in water electrolysis, particularly in alkaline systems. This enhancement is attributed to several factors that collectively contribute to improved electrolyzer performance and increased hydrogen yield.
One of the primary ways titanium mesh enhances efficiency is through its role in catalyst support. The high surface area provided by the mesh structure allows for a greater loading of catalytic materials. This increased catalyst surface area translates to more active sites for the hydrogen evolution reaction (HER) at the cathode and the oxygen evolution reaction (OER) at the anode. As a result, the overall reaction kinetics are improved, leading to higher hydrogen production rates at lower overpotentials.
The corrosion resistance of titanium mesh also plays a crucial role in maintaining long-term efficiency. In the harsh alkaline environment of water electrolysis, many electrode materials would degrade over time, leading to decreased performance. However, titanium's excellent corrosion resistance ensures that the electrode structure remains stable, maintaining its catalytic activity and efficiency over extended periods of operation. This stability is crucial for industrial applications where consistent, long-term performance is essential.
Furthermore, the porous nature of titanium mesh contributes to efficient gas evolution and separation. As hydrogen is produced at the cathode, the mesh structure allows for easy release and collection of the gas. This efficient gas management prevents the accumulation of hydrogen bubbles on the electrode surface, which could otherwise impede the electrolysis process. The result is a more continuous and efficient hydrogen production process.
The electrical properties of titanium mesh also contribute to enhanced efficiency. While titanium is not the most conductive metal, its conductivity is sufficient for effective electron transfer in electrolysis applications. The mesh structure ensures good electrical contact throughout the electrode, minimizing resistance losses and contributing to overall system efficiency.
Another aspect where titanium mesh enhances efficiency is through improved mass transport. The open structure of the mesh allows for efficient circulation of the electrolyte solution, ensuring a constant supply of reactants to the electrode surface. This enhanced mass transport helps to prevent concentration polarization, a phenomenon that can limit the rate of electrolysis reactions.
The mechanical strength of titanium mesh also contributes to efficiency enhancements. In large-scale electrolyzers, the electrode structure must withstand significant mechanical stresses due to gas evolution and fluid dynamics. The robust nature of titanium mesh ensures that the electrode maintains its structural integrity under these conditions, preventing performance degradation that could result from electrode deformation or damage.
Moreover, the use of titanium mesh allows for the development of advanced electrode designs that can further enhance efficiency. For example, gradient porosity structures or hierarchical mesh designs can be created to optimize both mass transport and catalytic activity. These advanced designs, made possible by the versatility of titanium mesh, can lead to significant improvements in hydrogen production efficiency.

In conclusion, titanium mesh is a versatile and highly effective material for use in alkaline water electrolysis systems. Its unique combination of properties – including corrosion resistance, high surface area, and mechanical strength – make it an ideal choice for electrode-diaphragm assemblies. The use of titanium mesh contributes to improved electrode performance, enhanced system efficiency, and increased hydrogen production rates. As the demand for clean hydrogen production continues to grow, the role of titanium mesh in advancing electrolysis technology is likely to become even more significant. Ongoing research and development in this field promise to unlock even greater potential for titanium mesh in the future of hydrogen energy.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Schalenbach, M., et al. (2018). "Alkaline Water Electrolysis: A Review." Journal of The Electrochemical Society, 165(11), J3041-J3051.
2. Marini, S., et al. (2012). "Advanced alkaline water electrolysis." Electrochimica Acta, 82, 384-391.
3. Zeng, K., & Zhang, D. (2010). "Recent progress in alkaline water electrolysis for hydrogen production and applications." Progress in Energy and Combustion Science, 36(3), 307-326.
4. Phillips, R., & Dunnill, C. W. (2016). "Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas." RSC Advances, 6(102), 100643-100651.
5. Ursua, A., et al. (2012). "Hydrogen Production From Water Electrolysis: Current Status and Future Trends." Proceedings of the IEEE, 100(2), 410-426.
6. Pletcher, D., & Li, X. (2011). "Prospects for alkaline zero gap water electrolysers for hydrogen production." International Journal of Hydrogen Energy, 36(23), 15089-15104.
7. Carmo, M., et al. (2013). "A comprehensive review on PEM water electrolysis." International Journal of Hydrogen Energy, 38(12), 4901-4934.
8. Chakik, F. E., et al. (2017). "Alkaline water electrolysis with Ni-based electrodes: Electrode activation with cerium sulfate." International Journal of Hydrogen Energy, 42(2), 1467-1484.
9. Kotrel, S., & Bräuninger, S. (2018). "Industrial Electrocatalysis." In Handbook of Heterogeneous Catalysis, 1-31.
10. Wang, M., et al. (2014). "The intensification technologies to water electrolysis for hydrogen production – A review." Renewable and Sustainable Energy Reviews, 29, 573-588.
Related Industry Knowledge
- Why are Titanium Electrodes Specifically Used for Ballast Water Management?
- Why is Titanium Used as Anode in Electrolysis of Brine?
- Electrochemical Essentials: The Comprehensive Guide to Anode Plates
- The Power of Splitting Water: An In-Depth Look at Alkaline Water Electrolyzers
- Why Should You Consider Electrodeposited Titanium Electrodes for Copper Plating?
- Which electrolyzer is best for hydrogen production?








Electrolyzers.webp)