- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Titanium electrodes have emerged as a promising technology in the field of water treatment, particularly for large-scale applications. As communities worldwide face increasing challenges in providing clean and safe drinking water, innovative solutions are needed to meet growing demands. Titanium electrodes offer several advantages, including durability, efficiency, and versatility, making them an attractive option for water treatment facilities. This blog post will explore the potential of titanium electrodes in large-scale water treatment and address some common questions about their use in drinking water disinfection.
How do titanium electrodes compare to traditional disinfection methods?
When it comes to water treatment, traditional disinfection methods such as chlorination and UV irradiation have been widely used for decades. However, titanium electrodes offer several advantages that make them a compelling alternative or complementary technology for large-scale water treatment.
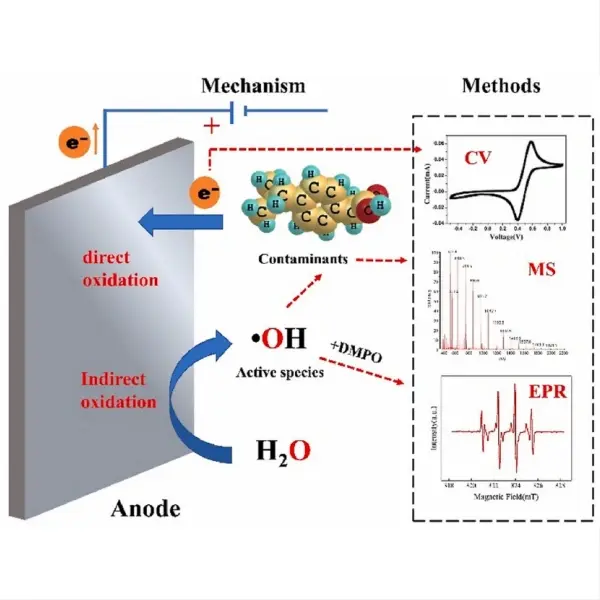
One of the primary benefits of titanium electrodes is their ability to generate powerful oxidizing agents directly in the water. Through a process called electrochemical oxidation, titanium electrodes can produce hydroxyl radicals, ozone, and other reactive oxygen species that effectively eliminate a wide range of contaminants, including bacteria, viruses, and organic pollutants. This in-situ generation of disinfectants eliminates the need for chemical storage and handling, reducing operational risks and costs associated with traditional chlorination methods.
Compared to UV irradiation, titanium electrodes offer the advantage of treating water with higher turbidity or dissolved organic matter content. While UV light can be blocked or scattered by particles in the water, electrochemical oxidation can penetrate and treat water more effectively in these challenging conditions.
Furthermore, titanium electrodes can address a broader spectrum of water quality issues beyond just disinfection. They have shown effectiveness in removing emerging contaminants such as pharmaceuticals, personal care products, and endocrine disruptors, which are increasingly becoming a concern in water supplies worldwide.
However, it's important to note that titanium electrodes are not without limitations. The initial investment cost for implementing an electrochemical system can be higher than traditional disinfection methods. Additionally, the energy consumption of electrochemical systems needs to be carefully managed to ensure cost-effectiveness, especially for large-scale operations.
Despite these challenges, many water treatment facilities are finding that the long-term benefits of titanium electrodes, including reduced chemical usage, improved water quality, and lower maintenance requirements, outweigh the initial costs. As technology continues to advance and economies of scale are realized, the competitiveness of titanium electrode systems is likely to improve further.
What are the environmental impacts of using titanium electrodes for water treatment?
As environmental concerns continue to shape water treatment practices, it's crucial to examine the ecological footprint of titanium electrodes in large-scale applications. The environmental impacts of using titanium electrodes for water treatment are multifaceted and generally considered to be favorable when compared to traditional methods.
One of the most significant environmental benefits of titanium electrodes is the reduction in chemical usage. Traditional water treatment often relies heavily on chlorine and other chemical disinfectants, which can lead to the formation of harmful disinfection by-products (DBPs) when they react with organic matter in the water. These DBPs, such as trihalomethanes and haloacetic acids, are known to have potential health and environmental risks. Titanium electrodes, by contrast, can achieve disinfection without the addition of chemicals, significantly reducing the potential for DBP formation.
Additionally, the durability and longevity of titanium electrodes contribute to their environmental sustainability. Titanium is highly resistant to corrosion and degradation, which means that electrodes can remain in service for extended periods without needing replacement. This longevity reduces the environmental impact associated with the production and disposal of treatment equipment.
However, it's important to consider the energy requirements of electrochemical systems. The process of electrolysis does consume electricity, and the environmental impact of this energy use depends largely on the source of power. In regions where renewable energy is prevalent, the carbon footprint of titanium electrode systems can be quite low. Conversely, in areas heavily reliant on fossil fuels for electricity generation, the indirect emissions from energy consumption need to be carefully evaluated.
Another environmental consideration is the potential for by-product formation during the electrochemical process. While titanium electrodes generally produce fewer harmful by-products than chemical disinfection methods, some studies have indicated the formation of trace amounts of chlorate and perchlorate ions, particularly when treating water with high chloride content. These by-products are regulated in many jurisdictions due to potential health effects, and their formation needs to be monitored and controlled.
On the positive side, titanium electrode systems have shown promise in addressing emerging environmental challenges. For instance, they have demonstrated effectiveness in removing micropollutants such as pharmaceuticals and personal care products from water, which are not always adequately addressed by conventional treatment methods. This capability can help prevent these contaminants from entering aquatic ecosystems and potentially disrupting wildlife.
Furthermore, the compact nature of electrochemical systems can lead to reduced land use for water treatment facilities, potentially preserving natural habitats or allowing for more efficient urban planning. The modular and scalable nature of these systems also allows for more flexible and targeted treatment approaches, potentially reducing overall resource consumption.
As research continues and technology advances, the environmental profile of titanium electrode systems is likely to improve further. Innovations in electrode design, system optimization, and integration with renewable energy sources are all areas of active development that could enhance the environmental sustainability of this technology.
Can titanium electrodes effectively remove emerging contaminants from drinking water?
The ability of water treatment systems to address emerging contaminants is becoming increasingly important as our understanding of water quality and its impact on human health evolves. Titanium electrodes have shown significant promise in this area, offering a versatile solution for removing a wide range of emerging contaminants from drinking water.
Emerging contaminants, also known as contaminants of emerging concern (CECs), include a diverse group of chemicals such as pharmaceuticals, personal care products, endocrine disruptors, and industrial chemicals. These substances are often present in water sources at very low concentrations but can potentially have adverse effects on human health and the environment, even at these trace levels.
Titanium electrodes, when used in advanced oxidation processes (AOPs), have demonstrated remarkable effectiveness in degrading many of these emerging contaminants. The electrochemical process generates highly reactive species such as hydroxyl radicals, which can break down complex organic molecules into simpler, less harmful compounds. This capability is particularly valuable for treating persistent organic pollutants that are resistant to conventional treatment methods.
Studies have shown that titanium electrode systems can effectively remove a variety of pharmaceuticals from water, including antibiotics, analgesics, and hormones. For example, research has demonstrated high removal rates for compounds like carbamazepine, diclofenac, and sulfamethoxazole, which are commonly found in wastewater and can persist through traditional treatment processes.
Furthermore, titanium electrodes have shown promise in addressing perfluoroalkyl and polyfluoroalkyl substances (PFAS), a group of man-made chemicals that have gained significant attention due to their persistence in the environment and potential health effects. While PFAS removal remains challenging for many conventional treatment technologies, electrochemical oxidation using titanium electrodes has demonstrated the ability to degrade these compounds effectively.
The versatility of titanium electrode systems allows for the treatment of a broad spectrum of emerging contaminants simultaneously, which is a significant advantage over more targeted treatment methods. This comprehensive approach can help water treatment facilities adapt to evolving water quality challenges and regulatory requirements without the need for frequent system upgrades.
However, it's important to note that the effectiveness of titanium electrodes in removing emerging contaminants can be influenced by various factors, including the specific contaminants present, their concentrations, water chemistry, and treatment conditions. Optimizing the electrochemical process for a particular water source and contaminant profile often requires careful system design and operational control.
Moreover, while titanium electrodes can effectively degrade many emerging contaminants, it's crucial to consider the potential formation of transformation products. In some cases, the breakdown of complex molecules can result in the formation of intermediate compounds, which may also need to be monitored and addressed.
As research in this field continues to advance, the potential of titanium electrodes for removing emerging contaminants is likely to expand further. Ongoing studies are exploring new electrode materials, coatings, and configurations to enhance treatment efficiency and selectivity. Additionally, the integration of electrochemical systems with other treatment technologies, such as membrane filtration or biological processes, shows promise for creating more comprehensive and efficient water treatment solutions.
In conclusion, titanium electrodes offer a powerful and versatile tool for large-scale water treatment, particularly in addressing the challenges of drinking water disinfection and emerging contaminant removal. While there are considerations regarding energy consumption and initial costs, the benefits in terms of treatment effectiveness, reduced chemical usage, and environmental impact make titanium electrodes a promising technology for the future of water treatment. As research continues and technology advances, we can expect to see wider adoption of titanium electrode systems in water treatment facilities worldwide, contributing to the provision of cleaner, safer drinking water for communities.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
2. Radjenovic, J., & Sedlak, D. L. (2015). Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environmental Science & Technology, 49(19), 11292-11302.
3. Chaplin, B. P. (2014). Critical review of electrochemical advanced oxidation processes for water treatment applications. Environmental Science: Processes & Impacts, 16(6), 1182-1203.
4. Sirés, I., Brillas, E., Oturan, M. A., Rodrigo, M. A., & Panizza, M. (2014). Electrochemical advanced oxidation processes: today and tomorrow. A review. Environmental Science and Pollution Research, 21(14), 8336-8367.
5. Ganiyu, S. O., Martínez-Huitle, C. A., & Rodrigo, M. A. (2020). Renewable energies driven electrochemical wastewater/soil decontamination technologies: A critical review of fundamental concepts and applications. Applied Catalysis B: Environmental, 270, 118857.
6. Trellu, C., Mousset, E., Pechaud, Y., Huguenot, D., van Hullebusch, E. D., Esposito, G., & Oturan, M. A. (2016). Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. Journal of Hazardous Materials, 306, 149-174.
7. Särkkä, H., Bhatnagar, A., & Sillanpää, M. (2015). Recent developments of electro-oxidation in water treatment—A review. Journal of Electroanalytical Chemistry, 754, 46-56.
8. Garcia-Segura, S., Ocon, J. D., & Chong, M. N. (2018). Electrochemical oxidation remediation of real wastewater effluents—A review. Process Safety and Environmental Protection, 113, 48-67.
9. Feng, L., van Hullebusch, E. D., Rodrigo, M. A., Esposito, G., & Oturan, M. A. (2013). Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chemical Engineering Journal, 228, 944-964.
10. Moreira, F. C., Boaventura, R. A., Brillas, E., & Vilar, V. J. (2017). Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Applied Catalysis B: Environmental, 202, 217-261.