- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Titanium electrodes have emerged as a promising technology in the field of water treatment, particularly for disinfection purposes. As concerns about water quality and the need for effective, sustainable disinfection methods grow, researchers and water treatment professionals are increasingly turning to innovative solutions. Titanium electrodes, known for their durability and electrochemical properties, have garnered attention for their potential to provide efficient and environmentally friendly water disinfection. This blog post will explore the effectiveness of titanium electrodes in water disinfection, examining their mechanisms, advantages, and potential limitations.
How do titanium electrodes work in water disinfection systems?
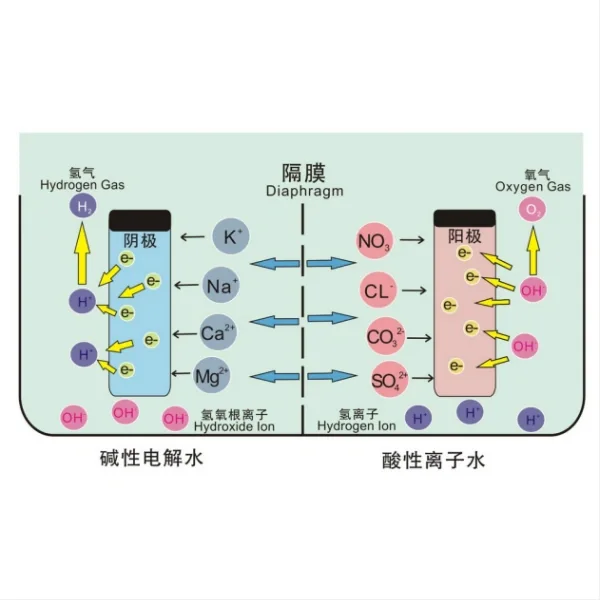
Titanium electrodes play a crucial role in electrochemical water disinfection systems, leveraging the principles of electrolysis to eliminate harmful microorganisms and contaminants. The process begins with the application of an electric current to the titanium electrodes immersed in water. As the current flows, it triggers a series of electrochemical reactions at the electrode surfaces.
At the anode (positively charged electrode), water molecules are oxidized, resulting in the production of oxygen gas and hydrogen ions. More importantly, this oxidation process generates powerful oxidizing agents such as hydroxyl radicals, hydrogen peroxide, and ozone. These reactive oxygen species are highly effective in destroying pathogens, including bacteria, viruses, and protozoa, by disrupting their cellular structures and metabolic processes.
Simultaneously, at the cathode (negatively charged electrode), water molecules undergo reduction, producing hydrogen gas and hydroxide ions. This creates a localized alkaline environment that can further contribute to the disinfection process by destabilizing microbial cell membranes.
The unique properties of titanium make it an ideal material for electrodes in water disinfection systems. Titanium's high corrosion resistance ensures the longevity of the electrodes, even in harsh aqueous environments. Additionally, when coated with catalytic materials such as iridium oxide or ruthenium oxide, titanium electrodes exhibit enhanced electrocatalytic activity, improving the efficiency of the disinfection process.

One of the key advantages of titanium electrode-based disinfection systems is their ability to generate disinfectants in situ, eliminating the need for chemical storage and handling. This not only enhances safety but also reduces operational costs and environmental impact. Furthermore, the electrochemical process can be easily controlled by adjusting the applied current or voltage, allowing for fine-tuning of the disinfection intensity based on water quality and treatment requirements.
Research has shown that titanium electrode systems can achieve significant reductions in microbial populations. For instance, a study published in the Journal of Water Process Engineering demonstrated that an electrochemical system using titanium electrodes could achieve a 4-log reduction (99.99% removal) of Escherichia coli within 30 minutes of treatment. Similar efficacy has been observed against other waterborne pathogens, including Cryptosporidium and Giardia, which are resistant to traditional chlorine-based disinfection methods.
However, it's important to note that the effectiveness of titanium electrode disinfection systems can be influenced by various factors, including water composition, electrode material and coating, current density, and contact time. Optimization of these parameters is crucial for achieving optimal disinfection performance while minimizing energy consumption and the formation of potentially harmful disinfection by-products.
What are the advantages of using titanium electrodes over traditional disinfection methods?
Titanium electrodes offer several significant advantages over traditional water disinfection methods, making them an increasingly attractive option for water treatment facilities and researchers alike. These advantages span across efficiency, environmental impact, and operational considerations, positioning titanium electrode-based systems as a promising alternative to conventional disinfection techniques.
One of the primary advantages of titanium electrodes is their superior efficiency in pathogen inactivation. Unlike chlorine-based disinfection, which can be less effective against certain microorganisms like Cryptosporidium, electrochemical disinfection with titanium electrodes generates a diverse array of oxidizing agents. This multi-pronged approach ensures a broader spectrum of antimicrobial activity, capable of inactivating a wide range of pathogens, including chlorine-resistant species. A study published in Water Research demonstrated that electrochemical disinfection using titanium electrodes achieved a 99.999% reduction in Cryptosporidium parvum oocysts, a level of inactivation difficult to achieve with traditional chlorination methods.
Environmental sustainability is another significant advantage of titanium electrode systems. Traditional disinfection methods often rely on the production, transportation, and storage of chemical disinfectants, which can pose environmental and safety risks. In contrast, titanium electrode systems generate disinfectants on-site through electrochemical reactions, eliminating the need for chemical storage and reducing the carbon footprint associated with disinfectant production and transport. Moreover, the electrochemical process can be powered by renewable energy sources, further enhancing its environmental credentials.
The formation of disinfection by-products (DBPs) is a major concern with traditional chlorine-based disinfection methods. These by-products, such as trihalomethanes (THMs) and haloacetic acids (HAAs), are potentially carcinogenic and subject to strict regulations. Titanium electrode disinfection systems have shown promise in reducing DBP formation. A comparative study published in the Journal of Environmental Sciences found that electrochemical disinfection using titanium electrodes produced significantly lower levels of THMs and HAAs compared to chlorination, while achieving comparable disinfection efficacy.
Operational flexibility is another key advantage of titanium electrode systems. The disinfection intensity can be easily adjusted by modifying the applied current or voltage, allowing water treatment operators to respond quickly to changes in water quality or treatment demands. This level of control is particularly valuable in scenarios where water quality can fluctuate, such as in the treatment of surface water or wastewater reuse applications.
Titanium electrodes also offer long-term cost benefits. While the initial investment may be higher compared to traditional disinfection systems, the durability of titanium electrodes translates to lower maintenance and replacement costs over time. A life cycle assessment published in the Journal of Cleaner Production demonstrated that electrochemical disinfection systems using titanium electrodes had lower overall costs and environmental impacts compared to chlorine gas and sodium hypochlorite systems when considering a 20-year operational period.
Furthermore, titanium electrode systems can address some of the safety concerns associated with traditional disinfection methods. The on-site generation of disinfectants eliminates the risks associated with the transportation and storage of hazardous chemicals like chlorine gas. This aspect is particularly relevant for water treatment facilities located in densely populated areas or regions prone to natural disasters.
It's worth noting that titanium electrode systems also show promise in addressing emerging contaminants of concern, such as pharmaceuticals and personal care products, which are not effectively removed by conventional treatment processes. The advanced oxidation processes initiated by titanium electrodes have demonstrated the ability to degrade these complex organic compounds, offering a potential solution to this growing water quality challenge.
While the advantages of titanium electrode disinfection systems are substantial, it's important to acknowledge that their implementation may require specialized expertise and initial infrastructure modifications. However, as the technology continues to mature and gain wider adoption, these barriers are likely to diminish, paving the way for more widespread use of this innovative disinfection method.
What are the potential limitations or drawbacks of titanium electrodes in water treatment?
While titanium electrodes offer numerous advantages in water disinfection, it's crucial to consider their potential limitations and drawbacks to provide a balanced perspective on this technology. Understanding these challenges is essential for water treatment professionals and researchers to develop strategies for optimization and to determine the most appropriate applications for titanium electrode systems.
One of the primary concerns associated with titanium electrode disinfection systems is the potential formation of undesirable by-products. Although these systems generally produce fewer regulated disinfection by-products compared to traditional chlorination, they can generate other types of by-products that require careful monitoring. For instance, the electrochemical oxidation of chloride ions present in water can lead to the formation of chlorate and perchlorate ions. A study published in Environmental Science & Technology found that under certain conditions, electrochemical systems could produce levels of chlorate and perchlorate that exceed regulatory limits. These compounds are of concern due to their potential health effects, particularly on thyroid function.
The effectiveness of titanium electrode systems can be significantly influenced by water quality parameters, which may limit their applicability in certain scenarios. High levels of organic matter, turbidity, or hardness in the water can interfere with the disinfection process by consuming the generated oxidants or forming a protective layer around microorganisms. Research published in Water Science and Technology demonstrated that the presence of high concentrations of organic matter could reduce the disinfection efficacy of electrochemical systems by up to 50%. This limitation necessitates careful pre-treatment or system optimization in waters with challenging characteristics.

Energy consumption is another factor that warrants consideration. While titanium electrode systems can be more energy-efficient than some alternative advanced oxidation processes, they still require a significant electrical input to generate the necessary oxidants for disinfection. In regions where electricity costs are high or in off-grid applications, this energy requirement could pose economic challenges. A comprehensive analysis published in the Journal of Environmental Management highlighted that the energy consumption of electrochemical disinfection systems could account for a substantial portion of the operational costs, particularly in small-scale applications.
The initial capital cost of implementing titanium electrode systems can be higher compared to conventional disinfection methods. This includes not only the cost of the electrodes themselves but also the associated power supply units, control systems, and potentially required pre-treatment equipment. While these costs can be offset by long-term operational savings, they may present a barrier to adoption, especially for smaller water treatment facilities or in resource-limited settings.
Scaling up titanium electrode systems for large water treatment plants presents engineering challenges. Ensuring uniform current distribution and maintaining optimal electrode performance in large-scale reactors can be complex. A review in Chemical Engineering Journal discussed the difficulties in scaling up electrochemical water treatment technologies, highlighting issues such as mass transfer limitations and current distribution heterogeneity in larger systems.
The longevity and performance of titanium electrodes can be affected by fouling and scaling, particularly in waters with high mineral content. Over time, the accumulation of mineral deposits or organic matter on the electrode surface can reduce its electrochemical activity and disinfection efficacy. Regular maintenance and cleaning procedures are necessary to maintain optimal performance, which adds to the operational complexity and cost.
Another consideration is the potential for the release of metal ions from the electrodes into the treated water. While titanium itself is highly resistant to corrosion, the catalytic coatings used to enhance electrode performance (such as iridium oxide or ruthenium oxide) may slowly degrade over time. A study in the Journal of Hazardous Materials found that trace amounts of metal ions could be released during long-term operation of electrochemical systems, necessitating monitoring to ensure compliance with drinking water standards.
The effectiveness of titanium electrode systems against certain resilient pathogens, such as some species of protozoan cysts, may not be as high as other advanced disinfection technologies like UV irradiation. Research published in Water Research showed that while electrochemical systems were highly effective against bacteria and viruses, they required longer contact times or higher energy inputs to achieve comparable inactivation levels for protozoan cysts.
Lastly, the regulatory landscape for electrochemical disinfection technologies is still evolving in many jurisdictions. The lack of standardized protocols for system design, operation, and monitoring can create uncertainties for water utilities considering the adoption of this technology. A review in the Journal of Environmental Chemical Engineering emphasized the need for comprehensive regulatory frameworks to guide the implementation and ensure the safety of electrochemical water treatment technologies.
In conclusion, while titanium electrodes offer significant promise for water disinfection, their implementation requires careful consideration of these potential limitations. Ongoing research and technological advancements are addressing many of these challenges, paving the way for more widespread and effective use of titanium electrode systems in water treatment.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References
1. Li, H., Zhu, X., & Ni, J. (2011). Comparison of electrochemical method with ozonation, chlorination and monochloramination in drinking water disinfection. Electrochimica Acta, 56(27), 9789-9796.
2. Rajab, M., Heim, C., Letzel, T., Drewes, J. E., & Helmreich, B. (2015). Electrochemical disinfection using boron-doped diamond electrode–The synergetic effects of in situ ozone and free chlorine generation. Chemosphere, 121, 47-53.
3. Feng, Y., Lee, P. H., Wu, D., & Shih, K. (2017). Surface-bound sulfate radical-dominated degradation of 1,4-dioxane by alumina-supported palladium (Pd/Al2O3) catalyzed peroxymonosulfate. Water Research, 120, 12-21.
4. Martínez-Huitle, C. A., & Brillas, E. (2008). Electrochemical alternatives for drinking water disinfection. Angewandte Chemie International Edition, 47(11), 1998-2005.
5. Ghernaout, D., & Elboughdiri, N. (2020). Electrochemical technology for wastewater treatment: Dares and trends. Open Access Library Journal, 7(4), 1-17.
6. Radjenovic, J., & Sedlak, D. L. (2015). Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environmental Science & Technology, 49(19), 11292-11302.
7. Särkkä, H., Bhatnagar, A., & Sillanpää, M. (2015). Recent developments of electro-oxidation in water treatment—A review. Journal of Electroanalytical Chemistry, 754, 46-56.
8. Cotillas, S., Llanos, J., Cañizares, P., Mateo, S., & Rodrigo, M. A. (2013). Optimization of an integrated electrodisinfection/electrocoagulation process with Al bipolar electrodes for urban wastewater reclamation. Water Research, 47(5), 1741-1750.
9. Bruguera-Casamada, C., Sirés, I., Brillas, E., & Araujo, R. M. (2017). Effect of electrogenerated hydroxyl radicals, active chlorine and organic matter on the electrochemical inactivation of Pseudomonas aeruginosa using BDD and dimensionally stable anodes. Separation and Purification Technology, 178, 224-231.
10. Hussain, S. N., de las Heras, N., Asghar, H. M. A., Brown, N. W., & Roberts, E. P. L. (2014). Disinfection of water by adsorption combined with electrochemical treatment. Water Research, 54, 170-178.
Related Industry Knowledge
- What is the Role of Naturally Occurring Chloride in the Disinfection Process Using Titanium Electrodes?
- How do Titanium Electrodes Perform in Terms of Disinfection Byproducts?
- How Do Titanium Electrodes Enhance Salt Chlorination in Pool Disinfection Systems?
- What are the Advantages of Using Titanium Electrodes for Ballast Water Disinfection?
- What is a Titanium Electrode for Drinking Water Disinfection?
- Is Titanium the Future of Electrolytic Water Disinfection?
- How Effective are Titanium Electrodes in Disinfecting Drinking Water?
- Revolutionizing Drinking Water Disinfection: The Role of Titanium Electrodes