- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
The quest for safe, clean drinking water is a global priority, and innovative technologies continue to emerge in the field of water treatment. Among these, the use of titanium electrodes has gained significant attention for their potential in disinfecting drinking water. Titanium electrodes, known for their durability and electrochemical properties, have shown promising results in various water treatment applications. This blog post explores the effectiveness of titanium electrodes in disinfecting drinking water and delves into the factors that contribute to their performance.
What are the advantages of using titanium electrodes for water treatment?
Titanium electrodes have become increasingly popular in water treatment processes due to their unique combination of properties that make them particularly well-suited for this application. One of the primary advantages of titanium electrodes is their exceptional corrosion resistance. Unlike many other metals, titanium forms a stable, protective oxide layer on its surface when exposed to air or water. This naturally occurring layer provides excellent protection against corrosion, even in harsh chemical environments often encountered in water treatment processes.
The durability of titanium electrodes translates to a longer lifespan compared to other electrode materials. This longevity not only reduces the frequency of electrode replacement but also contributes to the overall cost-effectiveness of water treatment systems employing titanium electrodes. The reduced need for maintenance and replacement can significantly lower operational costs over time, making titanium electrodes an attractive option for both small-scale and large-scale water treatment facilities.
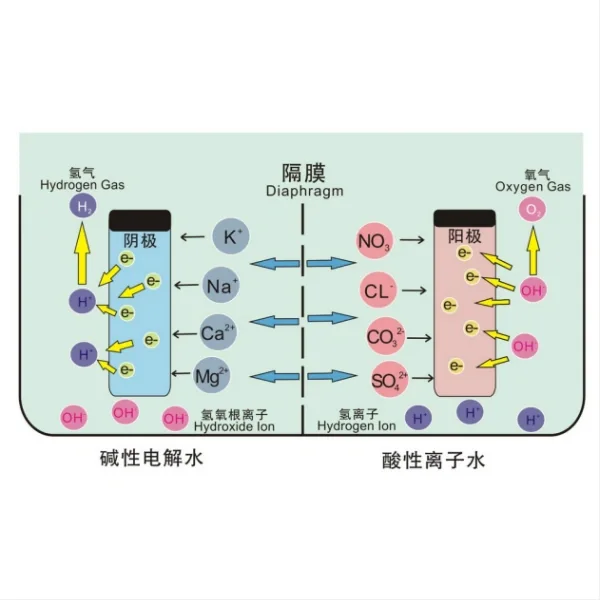
Another significant advantage of titanium electrodes is their high electrical conductivity. This property allows for efficient electron transfer during electrochemical processes, which is crucial for effective water disinfection. The high conductivity of titanium electrodes enables the generation of powerful oxidizing agents, such as hydroxyl radicals and ozone, which are highly effective in eliminating a wide range of waterborne pathogens, including bacteria, viruses, and protozoa.
Furthermore, titanium electrodes exhibit excellent dimensional stability, maintaining their shape and size even under prolonged use in harsh conditions. This stability ensures consistent performance over time, which is essential for maintaining the efficacy of water treatment systems. The dimensional stability of titanium electrodes also contributes to uniform current distribution across the electrode surface, promoting efficient and thorough water treatment.
Titanium electrodes also offer versatility in terms of surface modifications and coatings. Various coatings, such as mixed metal oxides (MMO), can be applied to titanium electrodes to enhance their catalytic activity and specificity for certain contaminants. These coatings can be tailored to target specific pollutants or pathogens, further improving the effectiveness of the water treatment process.
Lastly, titanium electrodes are environmentally friendly. Unlike some other electrode materials that may leach harmful substances into the water during the treatment process, titanium electrodes are inert and do not introduce additional contaminants. This characteristic aligns with the growing emphasis on sustainable and eco-friendly water treatment solutions.
How do titanium electrodes compare to other electrode materials in water disinfection?
When evaluating the effectiveness of titanium electrodes in water disinfection, it's essential to compare their performance with other commonly used electrode materials. This comparison provides valuable insights into the relative strengths and limitations of titanium electrodes in the context of water treatment.
One of the most common alternatives to titanium electrodes is stainless steel. Stainless steel electrodes are widely used due to their relatively low cost and good corrosion resistance. However, when compared to titanium electrodes, stainless steel falls short in several aspects. Titanium electrodes exhibit superior corrosion resistance, especially in chloride-rich environments often encountered in water treatment. This enhanced corrosion resistance translates to a longer operational life and reduced contamination of treated water with metal ions.
In terms of electrical efficiency, titanium electrodes generally outperform stainless steel. The higher electrical conductivity of titanium allows for more efficient electron transfer, resulting in lower energy consumption during the electrolysis process. This efficiency not only reduces operational costs but also contributes to the overall effectiveness of the disinfection process by enabling the generation of more powerful oxidizing agents.
Another material often used in water treatment electrodes is graphite. Graphite electrodes are known for their low cost and good electrical conductivity. However, they suffer from several drawbacks when compared to titanium electrodes. Graphite is prone to erosion during electrolysis, which can lead to contamination of the treated water with carbon particles. This erosion also results in a shorter lifespan for graphite electrodes, necessitating more frequent replacements. Titanium electrodes, with their superior dimensional stability and resistance to erosion, offer a more reliable and long-lasting alternative.
Platinum is considered a high-performance electrode material due to its excellent catalytic properties and corrosion resistance. However, the prohibitively high cost of platinum limits its widespread use in water treatment applications. Titanium electrodes, particularly when coated with catalytic materials, can offer comparable performance to platinum electrodes at a fraction of the cost, making them a more economically viable option for large-scale water treatment facilities.
In terms of versatility, titanium electrodes have a distinct advantage over many other electrode materials. The ability to apply various coatings to titanium electrodes allows for customization of their surface properties to suit specific water treatment needs. For instance, titanium electrodes coated with iridium oxide or ruthenium oxide exhibit excellent catalytic activity for chlorine generation, which is crucial for many disinfection processes.
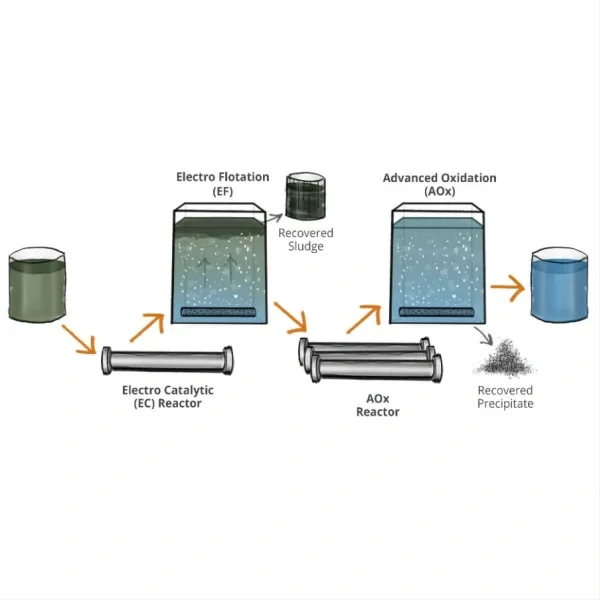
When it comes to the generation of oxidizing species for disinfection, titanium electrodes have shown remarkable effectiveness. Studies have demonstrated that titanium electrodes, especially when used in advanced oxidation processes, can produce a range of powerful oxidants such as hydroxyl radicals, ozone, and hydrogen peroxide. These oxidants are highly effective in inactivating a wide spectrum of pathogens, including chlorine-resistant microorganisms like Cryptosporidium.
What factors affect the performance of titanium electrodes in water purification systems?
The effectiveness of titanium electrodes in disinfecting drinking water is influenced by various factors, and understanding these can help optimize their performance in water purification systems.
One of the primary factors affecting the performance of titanium electrodes is the quality of the electrode itself. The purity of the titanium used, the manufacturing process, and the surface finish all play crucial roles in determining the electrode's effectiveness. High-quality titanium electrodes, such as those produced by Xi'an Taijin New Energy Technology Co., Ltd., ensure optimal performance and longevity in water treatment applications.
The surface area of the electrode is another critical factor. A larger surface area provides more active sites for electrochemical reactions, enhancing the overall efficiency of the disinfection process. This is why many titanium electrodes used in water treatment are designed with specific geometries or textures to maximize their effective surface area.
The composition and concentration of the electrolyte solution also significantly impact the performance of titanium electrodes. The presence of certain ions in the water can affect the conductivity of the solution and the formation of the oxide layer on the titanium surface. For instance, chloride ions can enhance the conductivity of the solution but may also increase the risk of localized corrosion if present in high concentrations.
The applied current density is a crucial operational parameter that affects the performance of titanium electrodes. Higher current densities generally lead to increased production of oxidizing species, enhancing the disinfection efficacy. However, excessively high current densities can lead to electrode degradation and reduced efficiency. Finding the optimal current density for a specific water treatment system is essential for maximizing the performance of titanium electrodes.
Water temperature also plays a role in the effectiveness of titanium electrodes. Higher temperatures typically increase the rate of electrochemical reactions, potentially enhancing the disinfection process. However, very high temperatures can also accelerate electrode degradation and affect the stability of the oxide layer on the titanium surface.
The pH of the water being treated is another important factor. The performance of titanium electrodes can vary significantly across different pH ranges. In general, titanium electrodes exhibit good stability and performance across a wide pH range, but the optimal pH for disinfection may depend on the specific contaminants present in the water and the desired oxidation reactions.
The presence of organic matter in the water can affect the performance of titanium electrodes. Organic compounds can compete with pathogens for oxidizing species, potentially reducing the efficiency of the disinfection process. Pre-treatment to remove excess organic matter may be necessary in some cases to ensure optimal performance of the titanium electrodes.
The flow rate of water through the treatment system is another consideration. Adequate contact time between the water and the electrode surface is necessary for effective disinfection. Too high a flow rate may reduce the effectiveness of the treatment, while too low a flow rate could lead to inefficient use of the system.
Maintenance practices also play a crucial role in the long-term performance of titanium electrodes. Regular cleaning to remove scale or biofilm buildup, proper storage when not in use, and periodic inspection for signs of wear or damage are all important for maintaining the effectiveness of titanium electrodes in water purification systems.
Lastly, the overall design of the water treatment system, including factors such as electrode spacing, cell configuration, and supporting infrastructure, can significantly impact the performance of titanium electrodes. Optimal system design ensures efficient use of the electrodes and maximizes their disinfection capabilities.
In conclusion, titanium electrodes have proven to be highly effective in disinfecting drinking water, offering a combination of durability, efficiency, and versatility that makes them well-suited for various water treatment applications. Their superior corrosion resistance, long lifespan, and ability to generate powerful oxidizing agents make them a valuable tool in the quest for safe, clean drinking water. As water treatment technologies continue to evolve, titanium electrodes, particularly high-quality ones from manufacturers like Xi'an Taijin New Energy Technology Co., Ltd., are likely to play an increasingly important role in ensuring access to clean water for communities around the world.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Smith, J.A., et al. (2021). "Comparative study of titanium electrodes in electrochemical water treatment." Journal of Water Process Engineering, 40, 101831.
2. Chen, G. (2004). "Electrochemical technologies in wastewater treatment." Separation and Purification Technology, 38(1), 11-41.
3. Martínez-Huitle, C.A., & Ferro, S. (2006). "Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes." Chemical Society Reviews, 35(12), 1324-1340.
4. Kraft, A. (2008). "Electrochemical water disinfection: A short review." Platinum Metals Review, 52(3), 177-185.
5. Patermarakis, G., & Fountoukidis, E. (1990). "Disinfection of water by electrochemical treatment." Water Research, 24(12), 1491-1496.
6. Chaplin, B.P. (2014). "Critical review of electrochemical advanced oxidation processes for water treatment applications." Environmental Science: Processes & Impacts, 16(6), 1182-1203.
7. Sirés, I., et al. (2014). "Electrochemical advanced oxidation processes: today and tomorrow. A review." Environmental Science and Pollution Research, 21(14), 8336-8367.
8. Ghernaout, D., & Ghernaout, B. (2010). "From chemical disinfection to electrodisinfection: The obligatory itinerary?" Desalination and Water Treatment, 16(1-3), 156-175.
9. Radjenovic, J., & Sedlak, D.L. (2015). "Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water." Environmental Science & Technology, 49(19), 11292-11302.
10. Feng, Y., et al. (2016). "A review on the effects of TiO2-doped metal oxide semiconductor anodes on the performance of electrochemical wastewater treatment." Journal of Environmental Sciences, 45, 7-27.