- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
What are the Advantages of Using Titanium Electrodes for Ballast Water Disinfection?
Ballast water disinfection is a critical process in maritime operations, aimed at preventing the spread of invasive aquatic species across different ecosystems. The use of titanium electrodes in this process has gained significant attention due to their unique properties and advantages. Titanium electrodes offer superior performance, longevity, and efficiency in ballast water treatment systems, making them an increasingly popular choice among shipowners and operators. This blog post will delve into the specific advantages of using titanium electrodes for ballast water disinfection and explore their impact on treatment efficiency, cost-effectiveness, and environmental sustainability.
How do titanium electrodes improve ballast water treatment efficiency?
Titanium electrodes play a crucial role in enhancing the efficiency of ballast water treatment systems through several key mechanisms. Firstly, titanium's exceptional corrosion resistance allows these electrodes to maintain their structural integrity and performance even in the harsh, saline environment of ballast water. This resistance to degradation ensures consistent and reliable disinfection over extended periods, reducing the need for frequent maintenance or replacement.
The high conductivity of titanium electrodes also contributes significantly to treatment efficiency. When an electric current is applied, titanium electrodes facilitate the efficient transfer of electrons, promoting the formation of powerful oxidizing agents such as hydroxyl radicals and chlorine species. These oxidants are highly effective in neutralizing a wide range of microorganisms, including bacteria, viruses, and larger organisms like algae and zooplankton.
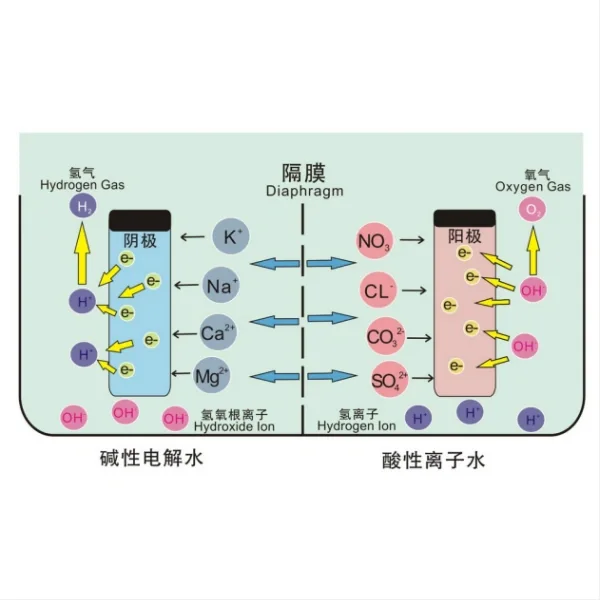
Moreover, titanium electrodes support a process known as electrochlorination, which is particularly effective in ballast water treatment. During electrochlorination, the titanium electrodes catalyze the conversion of naturally occurring chloride ions in seawater into hypochlorite, a potent disinfectant. This in-situ generation of disinfectants eliminates the need for storing and handling hazardous chemicals onboard, further streamlining the treatment process.
The surface properties of titanium electrodes also contribute to their efficiency. Titanium can be coated with specialized catalytic materials that enhance its electrochemical properties. These coatings can increase the electrode's surface area and catalytic activity, leading to more efficient oxidant production and, consequently, more effective disinfection.

Furthermore, titanium electrodes demonstrate excellent dimensional stability, maintaining their shape and size even under high current densities and prolonged use. This stability ensures consistent performance and uniform current distribution across the electrode surface, resulting in more predictable and reliable treatment outcomes.
The durability of titanium electrodes also translates to longer operational lifespans compared to electrodes made from other materials. This longevity not only improves the overall efficiency of the ballast water treatment system but also reduces downtime for maintenance and replacement, allowing for more consistent and uninterrupted treatment processes.
In summary, the use of titanium electrodes significantly improves ballast water treatment efficiency through their corrosion resistance, high conductivity, support for electrochlorination, enhanced surface properties, dimensional stability, and long operational lifespan. These characteristics collectively ensure a more effective, reliable, and sustainable approach to ballast water disinfection.
What are the cost implications of titanium electrodes in ballast water systems?
While the initial investment in titanium electrodes for ballast water treatment systems may be higher compared to alternatives, the long-term cost implications often favor this choice due to several factors. To fully understand the cost dynamics, it's essential to consider both the upfront expenses and the long-term operational costs associated with titanium electrodes.

The primary cost factor in implementing titanium electrodes is the higher initial purchase price. Titanium is a premium material with inherent value due to its rarity and the complex processes involved in its extraction and refinement. Consequently, the upfront cost of titanium electrodes can be significantly higher than that of electrodes made from more common materials like stainless steel or graphite.
However, the cost analysis becomes more favorable when considering the total cost of ownership over the system's lifetime. Titanium's exceptional durability and resistance to corrosion translate into a substantially longer operational lifespan. While other electrode materials may require frequent replacement due to wear, corrosion, or degradation, titanium electrodes can often last for many years, even in the harsh marine environment. This longevity significantly reduces the frequency and costs associated with electrode replacement, maintenance, and system downtime.
The efficiency of titanium electrodes in the ballast water treatment process also contributes to cost savings. Their high conductivity and electrochemical properties result in more effective disinfection, potentially reducing the energy requirements of the treatment system. This energy efficiency can lead to lower operational costs over time, especially considering the rising energy prices and the increasing focus on reducing fuel consumption in maritime operations.
Another cost-saving aspect is the reduced need for chemical storage and handling. Titanium electrodes' ability to support in-situ generation of disinfectants through processes like electrochlorination eliminates or significantly reduces the need to store and manage hazardous chemicals onboard. This not only simplifies logistics but also reduces costs associated with chemical purchases, storage facilities, and safety measures.
The dimensional stability and consistent performance of titanium electrodes also contribute to cost efficiency by ensuring predictable and reliable treatment outcomes. This reliability can help avoid costly scenarios such as failed port state control inspections or the need for emergency ballast water exchange, which can result in significant operational delays and associated expenses.
Maintenance costs are another area where titanium electrodes offer advantages. Their resistance to fouling and scaling means they require less frequent cleaning and maintenance compared to other electrode materials. This reduction in maintenance not only lowers direct costs but also minimizes system downtime, allowing for more continuous operation and potentially faster turnaround times in ports.
From a regulatory compliance perspective, the proven effectiveness of titanium electrodes in meeting stringent ballast water treatment standards can provide peace of mind to shipowners and operators. Compliance failures can result in substantial fines, operational restrictions, and reputational damage, all of which carry significant financial implications. The reliability of titanium electrodes in achieving consistent disinfection results helps mitigate these risks.
It's also worth considering the potential for future cost savings as technology advances. Ongoing research and development in electrode coatings and designs may further enhance the efficiency and lifespan of titanium electrodes, potentially offering even greater long-term cost benefits.
In conclusion, while the upfront costs of titanium electrodes for ballast water systems are higher, the long-term cost implications are often favorable. The extended lifespan, reduced maintenance needs, energy efficiency, and consistent performance contribute to lower total cost of ownership over the system's lifetime. As the maritime industry continues to prioritize efficiency and environmental compliance, the cost-effectiveness of titanium electrodes in ballast water treatment systems becomes increasingly apparent.
Are there any environmental benefits to using titanium electrodes for ballast water disinfection?
The use of titanium electrodes in ballast water disinfection systems offers several significant environmental benefits, aligning with the growing emphasis on sustainable maritime practices. These environmental advantages stem from the unique properties of titanium and the efficiency of the electrochemical treatment process it facilitates.
One of the primary environmental benefits is the reduction in chemical usage. Traditional ballast water treatment methods often rely on the addition of chemical disinfectants, which can have detrimental effects on marine ecosystems when discharged. Titanium electrodes, through their support of electrochlorination and other electrochemical processes, enable the in-situ generation of disinfectants using only the natural salts present in seawater. This approach significantly reduces the need for storing, handling, and discharging harmful chemicals, minimizing the risk of chemical pollution in ports and coastal areas.
The efficiency of titanium electrodes in the disinfection process also contributes to environmental protection. Their high conductivity and catalytic properties ensure more effective neutralization of harmful microorganisms and invasive species in ballast water. This thorough treatment helps prevent the introduction of non-native species into new ecosystems, which can have devastating effects on local biodiversity. By maintaining the ecological balance of marine environments, titanium electrode-based systems play a crucial role in preserving biodiversity and protecting sensitive aquatic habitats.
Energy efficiency is another key environmental benefit of titanium electrodes. Their superior conductivity and long-lasting performance mean that ballast water treatment systems can operate more efficiently, consuming less energy over time. This reduction in energy consumption translates to lower fuel usage and, consequently, reduced greenhouse gas emissions from ships. As the maritime industry faces increasing pressure to reduce its carbon footprint, the energy efficiency of titanium electrode systems becomes an important factor in sustainable shipping practices.
The durability and longevity of titanium electrodes also contribute to environmental sustainability. Their resistance to corrosion and wear means they need to be replaced less frequently than electrodes made from other materials. This longevity reduces the demand for raw materials and energy required for manufacturing and replacing electrodes, contributing to resource conservation and reducing the overall environmental impact of ballast water treatment systems.
Moreover, the effectiveness of titanium electrodes in treating ballast water without the need for temperature or salinity adjustments is environmentally beneficial. Some alternative treatment methods require heating or altering the chemistry of ballast water, which can be energy-intensive and potentially harmful to marine life when the treated water is discharged. Titanium electrode systems can operate effectively across a wide range of temperatures and salinities, minimizing the need for such adjustments and their associated environmental impacts.
The ability of titanium electrodes to support advanced oxidation processes (AOPs) offers additional environmental benefits. AOPs can break down a wide range of organic pollutants and emerging contaminants that may be present in ballast water, such as pharmaceutical residues or industrial chemicals. This capability enhances the overall water quality of treated ballast water, reducing the potential for these contaminants to impact marine ecosystems upon discharge.
Furthermore, the use of titanium electrodes aligns with the principles of green chemistry and engineering. The electrochemical processes they facilitate are inherently cleaner and more environmentally friendly than many chemical-based treatments. By minimizing the use of hazardous substances and reducing waste generation, titanium electrode systems contribute to the broader goals of environmental protection and sustainable development in the maritime sector.
In conclusion, the environmental benefits of using titanium electrodes for ballast water disinfection are numerous and significant. From reducing chemical pollution and protecting biodiversity to improving energy efficiency and supporting cleaner treatment processes, titanium electrodes play a crucial role in enhancing the environmental sustainability of maritime operations. As the industry continues to evolve towards more eco-friendly practices, the environmental advantages of titanium electrode-based ballast water treatment systems become increasingly valuable and relevant.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Tsolaki, E., & Diamadopoulos, E. (2010). Technologies for ballast water treatment: a review. Journal of Chemical Technology & Biotechnology, 85(1), 19-32.
2. Mouchtouri, V. A., Goutziana, G., Kremastinou, J., & Hadjichristodoulou, C. (2010). Emerging technologies for the disinfection of ballast water: A review. Journal of Marine Environmental Engineering, 9(3), 189-206.
3. Werschkun, B., Banerji, S., Basurko, O. C., David, M., Fuhr, F., Gollasch, S., ... & Kehrer, A. (2014). Emerging risks from ballast water treatment: The run-up to the International Ballast Water Management Convention. Chemosphere, 112, 256-266.
4. Čampara, L., Frančić, V., Maglić, L., & Hasanspahić, N. (2019). Overview of MARPOL ANNEX VI regulations for prevention of air pollution from marine diesel engines. SHS Web of Conferences, 58, 01004.
5. Batista, W. R., Fernandes, F. C., Lopes, C. C., Lopes, R. S., Miller, W., & Ruiz, G. (2017). Which ballast water management system will you put aboard? Remnant anxieties: A mini-review. Environments, 4(3), 54.
6. Moreno-Andrés, J., Romero-Martínez, L., Acevedo-Merino, A., & Nebot, E. (2017). Determining disinfection efficiency on E. faecalis in saltwater by photolysis of H2O2: Implications for ballast water treatment. Chemical Engineering Journal, 316, 114-122.
7. Bradie, J., Broeg, K., Gianoli, C., He, J., Heitmüller, S., Lo Curto, A., ... & Veldhuis, M. (2018). A shipboard comparison of analytic methods for ballast water compliance monitoring. Journal of Sea Research, 133, 11-19.
8. Lacasa, E., Tsolaki, E., Sbokou, Z., Rodrigo, M. A., Mantzavinos, D., & Diamadopoulos, E. (2013). Electrochemical disinfection of simulated ballast water on conductive diamond electrodes. Chemical Engineering Journal, 223, 516-523.
9. Zhang, N., Ma, B., Li, J., & Zhang, Z. (2013). Factors affecting formation of chemical by-products during ballast water treatment based on an advanced oxidation process. Chemical Engineering Journal, 231, 427-433.
10. Pereira, N. N., & Brinati, H. L. (2012). Onshore ballast water treatment: A viable option for major ports. Marine Pollution Bulletin, 64(11), 2296-2304.
Related Industry Knowledge
- How Can an Electrodeposited Titanium Electrode Improve the Performance of Nickel-Cobalt Batteries?
- How do Titanium Electrodes Affect the PH Balance of Pool Water?
- Are There Any Safety Considerations When Using Titanium Electrodes for Ballast Water?
- What does a Ballast Water Titanium Electrode do?
- What are the Advantages of Using Titanium Electrodes for Ballast Water Management?
- Purifying the Seas: The Role of Titanium Electrodes in Ballast Water Treatment