- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
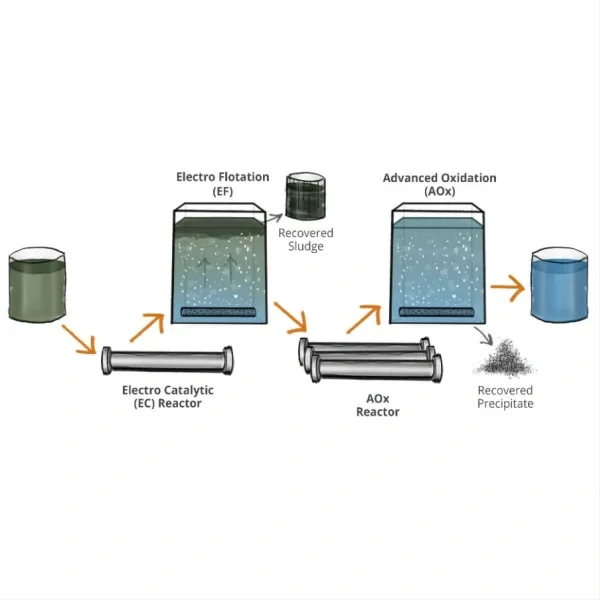
Titanium electrodes have gained significant attention in the field of water treatment, particularly for their potential in disinfection processes. As water quality and safety remain paramount concerns globally, researchers and engineers continually seek innovative solutions to enhance disinfection efficacy while minimizing harmful byproducts. Titanium electrodes, known for their durability and electrochemical properties, have emerged as a promising option in this arena. This blog post delves into the performance of titanium electrodes concerning disinfection byproducts, exploring their benefits, limitations, and overall impact on water treatment processes.
What are the advantages of using titanium electrodes in water disinfection?
Titanium electrodes offer several significant advantages in water disinfection processes, making them an increasingly popular choice in water treatment facilities worldwide. One of the primary benefits is their exceptional durability and resistance to corrosion. Unlike many other electrode materials, titanium can withstand harsh chemical environments and prolonged exposure to water without degrading, ensuring a longer operational lifespan and reduced maintenance costs.
The electrochemical properties of titanium electrodes also contribute to their effectiveness in water disinfection. When coated with suitable catalytic materials such as iridium oxide or ruthenium oxide, titanium electrodes exhibit high electrocatalytic activity. This enhanced activity allows for the efficient production of powerful oxidizing agents directly in the water, such as hydroxyl radicals, ozone, and hydrogen peroxide. These oxidants are highly effective in destroying a wide range of pathogens, including bacteria, viruses, and protozoa, without the need for additional chemical disinfectants.
Another advantage of titanium electrodes is their versatility in treating different types of water. They can be effectively used in various water sources, including drinking water, wastewater, and industrial process water. This adaptability makes titanium electrodes a valuable tool in addressing diverse water treatment challenges across different sectors.
Furthermore, the use of titanium electrodes in electrochemical disinfection processes often results in improved taste and odor of the treated water. Traditional chlorine-based disinfection methods can sometimes impart unpleasant tastes and odors to water, which can be a concern for consumers. Electrochemical disinfection using titanium electrodes typically avoids these issues, producing water that is not only safe but also more palatable.

The energy efficiency of titanium electrode systems is another notable advantage. When properly designed and operated, these systems can achieve high disinfection efficacy with relatively low energy input. This energy efficiency not only reduces operational costs but also aligns with the growing emphasis on sustainable and environmentally friendly water treatment solutions.
Lastly, titanium electrodes offer the potential for on-site disinfection, eliminating the need for transportation and storage of hazardous chemicals commonly used in traditional disinfection methods. This aspect enhances safety and reduces the environmental footprint associated with water treatment processes.
How do titanium electrodes compare to traditional chlorine-based disinfection methods?
The comparison between titanium electrode-based disinfection and traditional chlorine-based methods reveals several key differences in terms of efficacy, safety, and environmental impact. Chlorine has been the predominant disinfectant in water treatment for decades due to its effectiveness and relatively low cost. However, the use of titanium electrodes in electrochemical disinfection processes presents a compelling alternative that addresses some of the limitations associated with chlorine-based methods.
In terms of disinfection efficacy, both methods can effectively inactivate a wide range of waterborne pathogens. However, titanium electrode systems often demonstrate superior performance against certain resistant microorganisms. For instance, some protozoan parasites like Cryptosporidium, which are highly resistant to chlorine, can be more effectively inactivated by the strong oxidants produced by titanium electrodes. This broader spectrum of disinfection capability is particularly valuable in scenarios where water sources may contain diverse and potentially resistant microbial populations.
One of the most significant advantages of titanium electrode systems over chlorine-based methods is the reduced formation of harmful disinfection byproducts (DBPs). Chlorine reacts with organic matter in water to form trihalomethanes (THMs) and haloacetic acids (HAAs), which are regulated DBPs known to have potential health risks. In contrast, electrochemical disinfection using titanium electrodes typically produces fewer DBPs, particularly those regulated under current water quality standards. This reduction in DBP formation is a crucial factor in improving the overall safety profile of treated water.
The persistence of the disinfecting effect is another area where titanium electroes may offer advantages. Chlorine provides a residual disinfectant effect in the water distribution system, which helps prevent recontamination. While this residual effect is beneficial, it can also lead to continued DBP formation throughout the distribution network. Titanium electrode systems, on the other hand, produce short-lived but highly potent oxidants that provide immediate disinfection without leaving a persistent chemical residual. This characteristic can be advantageous in scenarios where long-term residual disinfection is not required or where the risk of DBP formation in the distribution system is a concern.
From an operational perspective, titanium electrode systems offer greater flexibility and control over the disinfection process. The disinfecting agents are produced on-demand and in-situ, allowing for rapid adjustments to treatment intensity based on water quality fluctuations or changing disinfection requirements. This adaptability contrasts with chlorine-based systems, where dosing adjustments may be slower and less precise.
Environmental considerations also favor titanium electrode systems in many cases. The production, transportation, and storage of chlorine and its compounds pose inherent risks and environmental concerns. Electrochemical disinfection eliminates these issues by generating disinfectants directly at the point of use. Additionally, the reduced formation of chlorinated DBPs contributes to lower environmental impact, particularly in aquatic ecosystems where treated water may be discharged.
However, it's important to note that the initial capital costs for implementing titanium electrode systems can be higher compared to traditional chlorine-based infrastructure. This factor may influence the choice of disinfection method, particularly for smaller water treatment facilities or in regions with limited resources.
Can titanium electrodes reduce the formation of harmful disinfection byproducts?
The potential of titanium electrodes to reduce the formation of harmful disinfection byproducts (DBPs) is one of their most significant advantages in water treatment. Traditional disinfection methods, particularly those using chlorine, are known to produce a range of DBPs, many of which have been associated with potential health risks. The use of titanium electrodes in electrochemical disinfection processes offers a promising approach to mitigating this issue.
The primary mechanism by which titanium electrodes can reduce DBP formation is through the nature of the oxidants they produce. When used in electrochemical systems, titanium electrodes typically generate highly reactive species such as hydroxyl radicals, ozone, and hydrogen peroxide. These oxidants are powerful disinfectants that can effectively inactivate pathogens without relying heavily on chlorine-based chemistry. As a result, the formation of common chlorinated DBPs like trihalomethanes (THMs) and haloacetic acids (HAAs) is significantly reduced.
Research has shown that electrochemical disinfection using titanium electrodes can achieve high levels of microbial inactivation while producing lower concentrations of regulated DBPs compared to conventional chlorination. For example, studies have demonstrated reductions in THM formation of up to 80% when using titanium electrode systems instead of traditional chlorine disinfection. This substantial decrease in DBP formation is a critical factor in improving the overall safety and quality of treated water.
Moreover, the electrochemical process allows for better control over the formation of other potentially harmful byproducts. By adjusting parameters such as current density, electrode material composition, and treatment time, it's possible to optimize the disinfection process to minimize the formation of undesirable compounds. This level of control is often more challenging to achieve with traditional chemical disinfection methods.
Another aspect of DBP reduction with titanium electrodes is related to the removal of DBP precursors. The strong oxidants generated by these electrodes can oxidize organic matter in the water, which are the primary precursors for many DBPs. By reducing the concentration of these precursors, the potential for DBP formation is further diminished, not only during the initial treatment but also in the subsequent stages of water distribution.
It's worth noting that while titanium electrodes can significantly reduce the formation of many common DBPs, they may potentially lead to the formation of other byproducts. However, these byproducts are typically less harmful and present in lower concentrations compared to those formed during chlorination. Ongoing research continues to investigate the full spectrum of byproducts associated with electrochemical disinfection to ensure comprehensive water quality and safety.
The ability of titanium electrode systems to reduce DBP formation while maintaining effective disinfection aligns well with increasingly stringent water quality regulations. As awareness of the potential health impacts of DBPs grows, many regulatory bodies are implementing stricter limits on allowable DBP concentrations in drinking water. Titanium electrode-based disinfection offers a viable solution for water treatment facilities to meet these evolving standards without compromising on disinfection efficacy.

In conclusion, titanium electrodes demonstrate significant potential in reducing the formation of harmful disinfection byproducts. By producing powerful oxidants that effectively disinfect water without relying on chlorine-based chemistry, these systems can achieve high levels of pathogen inactivation while minimizing the production of regulated DBPs. This capability, combined with the other advantages of titanium electrodes, makes them an increasingly attractive option for water treatment facilities seeking to enhance water quality and safety.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Li, H., Zhu, X., & Ni, J. (2011). Comparison of electrochemical method with ozonation, chlorination and monochloramination in drinking water disinfection. Electrochimica Acta, 56(27), 9789-9796.
2. Ghernaout, D., & Elboughdiri, N. (2020). Electrochemical technology for wastewater treatment: Dares and trends. Open Access Library Journal, 7(04), 1.
3. Martínez-Huitle, C. A., & Brillas, E. (2008). Electrochemical alternatives for drinking water disinfection. Angewandte Chemie International Edition, 47(11), 1998-2005.
4. Kraft, A. (2008). Electrochemical water disinfection: A short review. Platinum Metals Review, 52(3), 177-185.
5. Särkkä, H., Bhatnagar, A., & Sillanpää, M. (2015). Recent developments of electro-oxidation in water treatment—A review. Journal of Electroanalytical Chemistry, 754, 46-56.
6. Rajab, M., Heim, C., Letzel, T., Drewes, J. E., & Helmreich, B. (2015). Electrochemical disinfection using boron-doped diamond electrode–The synergetic effects of in situ ozone and free chlorine generation. Chemosphere, 121, 47-53.
7. Feng, Y., Lee, P. H., Wu, D., & Shih, K. (2017). Surface-bound sulfate radical-dominated degradation of 1,4-dioxane by alumina-supported palladium (Pd/Al2O3) catalyzed peroxymonosulfate. Water Research, 120, 12-21.
8. Radjenovic, J., & Sedlak, D. L. (2015). Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environmental Science & Technology, 49(19), 11292-11302.
9. Chaplin, B. P. (2014). Critical review of electrochemical advanced oxidation processes for water treatment applications. Environmental Science: Processes & Impacts, 16(6), 1182-1203.
10. Ganiyu, S. O., Martínez-Huitle, C. A., & Rodrigo, M. A. (2020). Renewable energies driven electrochemical wastewater/soil decontamination technologies: A critical review of fundamental concepts and applications. Applied Catalysis B: Environmental, 270, 118857.
Related Industry Knowledge
- How Do Anodes Facilitate the Removal of Ammonia Nitrogen from Water?
- Unlocking the Potential: The Science and Applications of Acidic Electrolytic Water
- Revolutionizing Drinking Water Disinfection: The Role of Titanium Electrodes
- Crystal Clear Waters: The Power of Titanium Electrodes in Pool Disinfection
- The Power of Splitting Water: An In-Depth Look at Alkaline Water Electrolyzers
- Crystal Clear Waters: Revolutionizing Pool Disinfection with Titanium Electrodes
- Purifying the Seas: The Role of Titanium Electrodes in Ballast Water Treatment