- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
How Effective are Titanium Electrodes at Eliminating Pathogens and Organic Contaminants?
Titanium electrodes have emerged as a promising technology in water treatment, particularly for their ability to eliminate pathogens and organic contaminants. These electrodes, often coated with various metal oxides, have shown remarkable efficiency in disinfecting water and removing harmful substances through electrochemical processes. As concerns about water quality continue to grow globally, the use of titanium electrodes in water treatment systems has garnered significant attention from researchers, environmental engineers, and policymakers alike.
How do titanium electrodes compare to traditional water disinfection methods?
When it comes to water disinfection, titanium electrodes offer several advantages over traditional methods such as chlorination or UV treatment. The electrochemical process initiated by titanium electrodes generates powerful oxidizing agents directly in the water, eliminating the need for additional chemical storage and handling. This in-situ generation of disinfectants not only improves safety but also ensures a more consistent and controllable treatment process.
One of the key benefits of titanium electrodes is their ability to produce a mix of oxidants, including hydroxyl radicals, ozone, and hydrogen peroxide. These oxidants are highly effective against a wide range of pathogens, including bacteria, viruses, and protozoa. Studies have shown that electrochemical disinfection using titanium electrodes can achieve log reductions of 3-5 for common waterborne pathogens, which is comparable to or better than traditional chlorination methods.
Moreover, titanium electrodes demonstrate superior effectiveness against chlorine-resistant microorganisms such as Cryptosporidium and Giardia. These parasites have proven challenging to eliminate with conventional disinfection methods, but the advanced oxidation processes initiated by titanium electrodes can effectively inactivate them. This broader spectrum of antimicrobial activity makes titanium electrodes particularly valuable in areas where waterborne parasitic infections are prevalent.
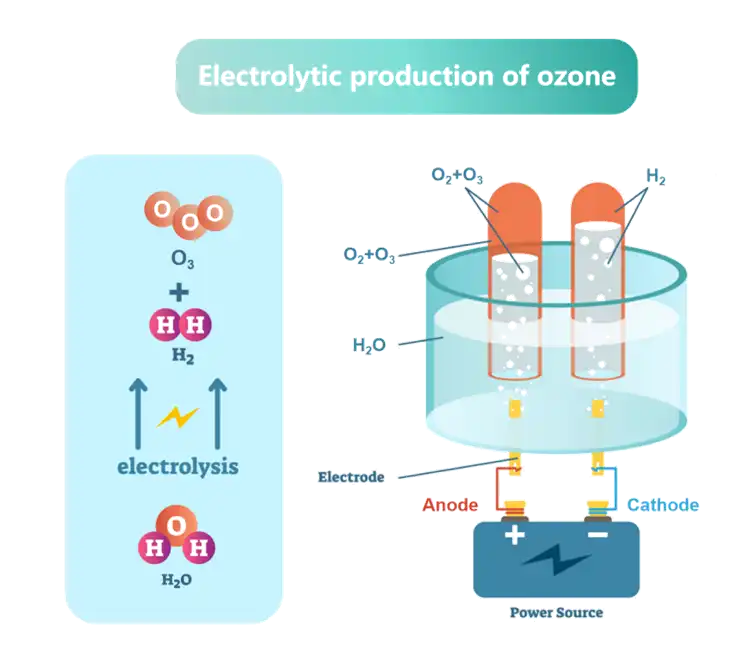
Another advantage of titanium electrodes is their ability to disinfect water without producing harmful disinfection by-products (DBPs) commonly associated with chlorination. Trihalomethanes (THMs) and haloacetic acids (HAAs), which are known carcinogens, are often formed when chlorine reacts with organic matter in water. Electrochemical disinfection using titanium electrodes significantly reduces the formation of these DBPs, leading to safer drinking water.
The energy efficiency of titanium electrode systems is also noteworthy. While the initial investment may be higher compared to traditional disinfection methods, the operational costs are often lower due to reduced chemical consumption and simplified maintenance procedures. Additionally, the process can be easily automated and integrated into existing water treatment facilities, making it a versatile solution for both small-scale and large-scale applications.
What role do titanium electrodes play in removing organic contaminants from water?
Titanium electrodes play a crucial role in removing organic contaminants from water through a process known as electrochemical advanced oxidation. This process is particularly effective against a wide range of persistent organic pollutants (POPs) that are resistant to conventional treatment methods. The electrochemical reactions initiated by titanium electrodes generate highly reactive species that can break down complex organic molecules into simpler, less harmful compounds.
One of the primary mechanisms by which titanium electrodes remove organic contaminants is through the production of hydroxyl radicals (•OH). These radicals are among the most powerful oxidizing agents known, capable of rapidly degrading organic pollutants such as pharmaceuticals, pesticides, and industrial chemicals. The non-selective nature of hydroxyl radicals allows them to attack a broad spectrum of organic contaminants, making the treatment process effective against both known and emerging pollutants.
Research has demonstrated the efficacy of titanium electrodes in removing various classes of organic contaminants. For instance, studies have shown removal efficiencies exceeding 90% for many pharmaceuticals and personal care products (PPCPs) that are increasingly found in water sources. These contaminants, which include antibiotics, hormones, and cosmetic ingredients, are often not effectively removed by conventional water treatment processes and can pose risks to aquatic ecosystems and human health.
Titanium electrodes have also proven effective in treating water contaminated with pesticides and herbicides. These agricultural chemicals are persistent in the environment and can contaminate surface and groundwater sources. Electrochemical oxidation using titanium electrodes has been shown to degrade many common pesticides, including atrazine and glyphosate, with high efficiency. This capability is particularly valuable in agricultural regions where pesticide contamination of water sources is a significant concern.
Industrial pollutants, such as dyes, solvents, and phenolic compounds, are another category of organic contaminants effectively treated by titanium electrodes. These substances are often challenging to remove using traditional biological treatment methods due to their complex molecular structures and potential toxicity to microorganisms. Electrochemical oxidation can break down these recalcitrant compounds into biodegradable intermediates or completely mineralize them to carbon dioxide and water.
The flexibility of titanium electrode systems allows for the treatment of water with varying levels of organic contamination. By adjusting parameters such as current density, electrode material, and electrolyte composition, the treatment process can be optimized for specific contaminants or water qualities. This adaptability makes titanium electrodes suitable for a wide range of applications, from treating highly contaminated industrial wastewater to polishing drinking water supplies.
Moreover, the electrochemical treatment process using titanium electrodes can be combined with other treatment technologies to create multi-barrier systems. For example, integrating electrochemical oxidation with membrane filtration or biological treatment can result in more comprehensive and efficient water purification processes. These hybrid systems can address a broader range of water quality issues and provide enhanced protection against both microbial and chemical contaminants.
Can titanium electrodes be used for large-scale drinking water disinfection systems?
The scalability of titanium electrode technology for drinking water disinfection has been a subject of significant research and development in recent years. As water treatment facilities seek more efficient and sustainable disinfection methods, the potential for using titanium electrodes in large-scale systems has garnered increasing attention. The question of whether titanium electrodes can be effectively implemented in large-scale drinking water disinfection systems is complex, involving considerations of efficiency, cost-effectiveness, and practical implementation.
One of the primary advantages of titanium electrodes for large-scale applications is their durability and longevity. Titanium, especially when coated with catalytic materials like mixed metal oxides, exhibits excellent resistance to corrosion and erosion. This durability translates to lower maintenance requirements and longer operational lifespans compared to other electrode materials. In large-scale systems where continuity of operation is critical, the robustness of titanium electrodes can significantly reduce downtime and replacement costs.
The modular nature of electrochemical systems also lends itself well to scalability. Titanium electrode arrays can be designed and configured to treat varying volumes of water, allowing for flexible implementation in treatment plants of different sizes. This modularity also facilitates easier upgrades and expansions of existing systems, as additional electrode units can be integrated to increase treatment capacity without major overhauls of the entire system.
Energy efficiency is another factor that supports the use of titanium electrodes in large-scale applications. While the initial energy input required for electrochemical disinfection may be higher compared to some traditional methods, the overall energy balance can be favorable when considering the elimination of chemical transportation, storage, and dosing systems. Additionally, ongoing research is focused on optimizing electrode designs and operational parameters to further improve energy efficiency, making the technology increasingly viable for large-scale implementation.
However, challenges remain in scaling up titanium electrode systems for drinking water disinfection. One of the primary concerns is the initial capital investment required for implementing electrochemical disinfection systems. The cost of titanium and specialized coatings can be significant, especially when outfitting large treatment facilities. While these costs may be offset by long-term operational savings, they can present a barrier to adoption for some municipalities or water utilities with limited budgets.
Another challenge lies in ensuring uniform treatment in large-volume systems. As the scale of treatment increases, maintaining consistent electric field distribution and oxidant generation throughout the treatment volume becomes more complex. Engineering solutions, such as optimized electrode geometries and flow patterns, are being developed to address these issues and ensure effective disinfection across large water volumes.
Regulatory considerations also play a role in the adoption of titanium electrode technology for large-scale drinking water disinfection. While electrochemical disinfection has been proven effective in numerous studies, regulatory frameworks in many regions are still catching up to this technology. Establishing clear guidelines and approval processes for electrochemical disinfection systems will be crucial for widespread implementation in municipal water treatment facilities.
Despite these challenges, several pilot projects and full-scale implementations have demonstrated the feasibility of using titanium electrodes for large-scale drinking water disinfection. For example, a water treatment plant in the Netherlands successfully implemented an electrochemical disinfection system using titanium electrodes to treat up to 1,400 m³/h of drinking water. The system has shown consistent performance in pathogen inactivation while reducing the formation of disinfection by-products.
As research continues and more data becomes available from large-scale implementations, the viability of titanium electrodes for drinking water disinfection is likely to increase. Ongoing advancements in electrode materials, system design, and operational optimization are continually improving the efficiency and cost-effectiveness of this technology. Furthermore, as concerns about chemical disinfection by-products and the environmental impact of traditional treatment methods grow, the appeal of electrochemical disinfection using titanium electrodes is expected to rise.
In conclusion, while challenges remain, the potential for using titanium electrodes in large-scale drinking water disinfection systems is promising. The technology offers significant advantages in terms of effectiveness, sustainability, and adaptability. As the water treatment industry continues to evolve and seek innovative solutions to meet growing global water demands, titanium electrode systems are likely to play an increasingly important role in ensuring safe and clean drinking water for large populations.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References
1. Martínez-Huitle, C. A., & Brillas, E. (2008). Electrochemical alternatives for drinking water disinfection. Angewandte Chemie International Edition, 47(11), 1998-2005.
2. Feng, Y., et al. (2016). Electrochemical technologies for wastewater treatment and resource reclamation. Environmental Science: Water Research & Technology, 2(5), 800-831.
3. Radjenovic, J., & Sedlak, D. L. (2015). Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environmental Science & Technology, 49(19), 11292-11302.
4. Särkkä, H., Bhatnagar, A., & Sillanpää, M. (2015). Recent developments of electro-oxidation in water treatment—A review. Journal of Electroanalytical Chemistry, 754, 46-56.
5. Ganiyu, S. O., Martínez-Huitle, C. A., & Rodrigo, M. A. (2020). Electrochemical advanced oxidation processes for the removal of organic pollutants in water and wastewater. Current Opinion in Electrochemistry, 22, 211-220.
6. Chaplin, B. P. (2014). Critical review of electrochemical advanced oxidation processes for water treatment applications. Environmental Science: Processes & Impacts, 16(6), 1182-1203.
7. Brillas, E., & Martínez-Huitle, C. A. (2015). Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Applied Catalysis B: Environmental, 166, 603-643.
8. Rajeshwar, K., et al. (2013). Electrochemical remediation of the environment. Journal of Applied Electrochemistry, 43(1), 1-11.
9. Garcia-Segura, S., et al. (2018). Electrochemical oxidation of organic pollutants for wastewater treatment: Current status and perspectives. Applied Catalysis B: Environmental, 236, 546-573.
10. Moreira, F. C., et al. (2017). Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Applied Catalysis B: Environmental, 202, 217-261.
Related Industry Knowledge
- Why Should You Consider Titanium Electrodes for Copper Plating?
- How Can Titanium Electrode Improve Nickel And Cobalt Electrodeposition Performance?
- What Industries Rely on DSA Anodes for Electrochemical Processes?
- What Factors Should Be Considered When Selecting a DSA Anode?
- What Is a Chlorine Generator Electrolyzer and How Does It Operate?
- What is a DSA Anode and How Does It Work?
- What Industries Benefit from Chlorine Generator Electrolyzers for On-Site Production?