- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Titanium electrodes play a crucial role in various electrochemical processes, including water treatment and ballast water management systems. These electrodes are prized for their exceptional corrosion resistance, durability, and electrical conductivity. In the context of ballast water treatment, titanium electrodes are utilized to generate disinfecting agents that eliminate harmful microorganisms and prevent the spread of invasive species through ships' ballast water. This blog post will delve into the specifics of titanium electrodes in ballast water treatment systems and address some common questions about their function and effectiveness.
How do titanium electrodes work in ballast water treatment systems?
Titanium electrodes are a key component in electrochemical ballast water treatment systems, which are designed to purify and disinfect ballast water before it's discharged from ships. These systems rely on the process of electrolysis to generate powerful oxidizing agents directly from the seawater itself, eliminating the need for additional chemicals to be stored onboard.
The process begins when ballast water flows through a chamber containing titanium electrodes. An electric current is passed through these electrodes, causing electrolysis of the water molecules. This reaction produces a mixture of oxidizing agents, primarily hypochlorite (OCl-) and hypochlorous acid (HOCl), which are highly effective at killing or inactivating a wide range of microorganisms, including bacteria, viruses, and larger organisms like plankton.
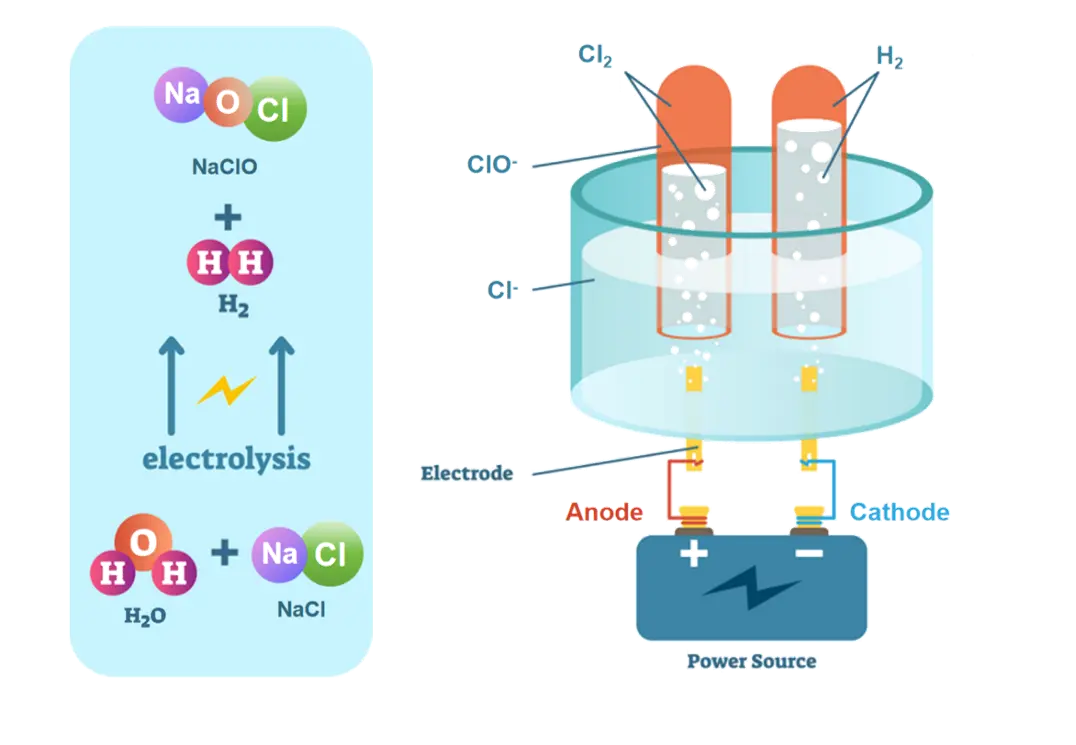
Titanium is the material of choice for these electrodes due to its unique properties. It forms a stable, protective oxide layer when exposed to air or water, making it highly resistant to corrosion even in the harsh marine environment. This corrosion resistance is crucial for maintaining the longevity and efficiency of the treatment system. Additionally, titanium has excellent electrical conductivity, which is essential for the efficient generation of the disinfecting agents.
The effectiveness of titanium electrodes in ballast water treatment systems is further enhanced by their ability to withstand high current densities without degradation. This allows for the rapid and efficient production of disinfecting agents, ensuring that large volumes of ballast water can be treated quickly to meet the operational needs of ships.
Moreover, titanium electrodes can be manufactured with specialized coatings or surface treatments to further improve their performance. For example, some electrodes are coated with mixed metal oxides (MMO) to enhance their catalytic properties and increase the production of oxidizing agents.
What are the advantages of using titanium electrodes over other materials?
The use of titanium electrodes in ballast water treatment systems offers several significant advantages over alternative materials, making them the preferred choice for many manufacturers and ship operators.
First and foremost, titanium's exceptional corrosion resistance is a major benefit in the marine environment. Seawater is highly corrosive due to its salt content and the presence of various minerals and microorganisms. Many metals would quickly deteriorate under these conditions, but titanium forms a stable, protective oxide layer that allows it to withstand this harsh environment for extended periods. This durability translates to lower maintenance requirements and longer service life for the treatment systems, reducing operational costs and downtime for ship operators.
Another advantage of titanium electrodes is their high electrical efficiency. Titanium has a relatively low electrical resistance, which means that less energy is wasted as heat during the electrolysis process. This efficiency is crucial for shipboard applications, where power consumption is a significant consideration. By minimizing energy losses, titanium electrodes help to reduce the overall power demands of the ballast water treatment system, contributing to improved fuel efficiency and lower operating costs for the vessel.
Titanium electrodes also offer excellent dimensional stability, maintaining their shape and size even under high current densities and prolonged use. This stability ensures consistent performance over time, which is essential for meeting stringent ballast water treatment standards. In contrast, electrodes made from less stable materials might deform or erode over time, leading to reduced efficiency and potentially compromising the treatment system's effectiveness.
The biocompatibility of titanium is another advantage, particularly in the context of environmental protection. Unlike some other electrode materials that might leach harmful substances into the treated water, titanium is biologically inert. This property ensures that the use of titanium electrodes does not introduce additional contaminants into the marine environment, aligning with the overall goal of ballast water treatment to protect aquatic ecosystems.
Furthermore, titanium electrodes can be manufactured with various surface modifications to enhance their performance. For instance, the application of mixed metal oxide (MMO) coatings can significantly increase the electrode's catalytic activity, improving the efficiency of oxidant generation. These coatings can be tailored to optimize the production of specific disinfecting agents, allowing for customization of the treatment process to meet particular water quality challenges or regulatory requirements.
Lastly, the long-term cost-effectiveness of titanium electrodes is a major advantage. While the initial investment in titanium electrodes may be higher compared to some alternative materials, their longevity and low maintenance requirements often result in lower total cost of ownership over the life of the ballast water treatment system. This economic benefit, combined with their superior performance characteristics, makes titanium electrodes an attractive choice for both system manufacturers and end-users.
How effective are titanium electrodes in eliminating invasive species from ballast water?
The effectiveness of titanium electrodes in eliminating invasive species from ballast water is a critical concern for the shipping industry and environmental regulators alike. Ballast water has been identified as a major vector for the spread of non-native aquatic organisms, which can have devastating effects on local ecosystems when introduced to new environments. The use of titanium electrodes in electrochemical ballast water treatment systems has shown promising results in addressing this challenge.
The primary mechanism by which titanium electrodes contribute to the elimination of invasive species is through the generation of powerful oxidizing agents. When an electric current is passed through titanium electrodes in seawater, it produces a mixture of disinfectants, primarily hypochlorite and hypochlorous acid. These compounds are highly effective at inactivating or killing a wide range of microorganisms, including bacteria, viruses, and larger organisms such as plankton and the larval stages of various marine species.
Research has shown that electrochemical systems using titanium electrodes can achieve high kill rates for a variety of organisms. For example, studies have demonstrated that these systems can effectively eliminate common ballast water contaminants such as Escherichia coli, Enterococcus faecalis, and Vibrio cholerae, as well as larger organisms like copepods and barnacle larvae. The oxidizing agents generated by the titanium electrodes disrupt cellular processes, damage cell membranes, and interfere with DNA replication, leading to the rapid death of these organisms.
One of the key advantages of using titanium electrodes for this purpose is the ability to generate disinfecting agents on-demand and in-situ. This eliminates the need for ships to carry large quantities of chemical disinfectants, which can be hazardous and require special handling and storage. The on-demand generation also ensures that the treatment process can be easily adjusted to accommodate varying water qualities and organism loads encountered in different ports around the world.
The effectiveness of titanium electrodes in ballast water treatment systems is further enhanced by their ability to operate consistently over long periods. The durability and corrosion resistance of titanium ensure that the electrodes maintain their performance characteristics even after extended use in seawater. This consistency is crucial for meeting the stringent discharge standards set by international regulations such as the International Maritime Organization's Ballast Water Management Convention.
However, it's important to note that the effectiveness of titanium electrodes in eliminating invasive species can be influenced by various factors. Water quality parameters such as temperature, salinity, and turbidity can affect the efficiency of oxidant generation and the overall treatment process. Additionally, some organisms may be more resistant to oxidative stress than others, requiring higher doses or longer contact times for effective inactivation.
To address these challenges, many ballast water treatment systems incorporating titanium electrodes are designed with multiple treatment stages or complementary technologies. For example, some systems combine electrochemical oxidation with filtration or UV irradiation to provide a multi-barrier approach to organism elimination. This comprehensive treatment strategy helps to ensure compliance with regulatory standards across a wide range of operating conditions and organism types.
Ongoing research and development in this field continue to improve the effectiveness of titanium electrodes in ballast water treatment. Advances in electrode design, surface coatings, and control systems are enhancing the efficiency and reliability of these systems. Furthermore, the development of real-time monitoring technologies is enabling more precise control of the treatment process, allowing for optimization of oxidant production based on actual water quality and organism load.
In conclusion, titanium electrodes have proven to be highly effective in eliminating invasive species from ballast water when used in properly designed and operated electrochemical treatment systems. Their ability to generate powerful disinfecting agents on-demand, combined with their durability and consistency, makes them a valuable tool in the global effort to prevent the spread of non-native aquatic organisms through maritime transport. As technology continues to advance and our understanding of invasive species biology improves, the role of titanium electrodes in protecting marine ecosystems is likely to become even more significant.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. International Maritime Organization. (2004). International Convention for the Control and Management of Ships' Ballast Water and Sediments.
2. Tsolaki, E., & Diamadopoulos, E. (2010). Technologies for ballast water treatment: a review. Journal of Chemical Technology & Biotechnology, 85(1), 19-32.
3. Lacasa, E., Tsolaki, E., Sbokou, Z., Rodrigo, M. A., Mantzavinos, D., & Diamadopoulos, E. (2013). Electrochemical disinfection of simulated ballast water on conductive diamond electrodes. Chemical Engineering Journal, 223, 516-523.
4. Nanayakkara, K. G. N., Alam, A. K. M. K., Zheng, Y. M., & Chen, J. P. (2012). A low-energy intensive electrochemical system for the eradication of Escherichia coli from ballast water: Process development, disinfection chemistry, and kinetics modeling. Marine Pollution Bulletin, 64(6), 1238-1245.
5. Kraft, A. (2008). Electrochemical water disinfection: A short review. Platinum Metals Review, 52(3), 177-185.
6. Drogui, P., Elmaleh, S., Rumeau, M., Bernard, C., & Rambaud, A. (2001). Oxidising and disinfecting by hydrogen peroxide produced in a two-electrode cell. Water Research, 35(13), 3235-3241.
7. Jeong, J., Kim, C., & Yoon, J. (2009). The effect of electrode material on the generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Research, 43(4), 895-901.
8. Moreno-Andrés, J., Romero-Martínez, L., Acevedo-Merino, A., & Nebot, E. (2017). Determining disinfection efficiency on E. faecalis in saltwater by photolysis of H2O2: Implications for ballast water treatment. Chemical Engineering Journal, 316, 703-715.
9. Zhang, Y., Chen, Y., Westerhoff, P., Hristovski, K., & Crittenden, J. C. (2008). Stability of commercial metal oxide nanoparticles in water. Water Research, 42(8-9), 2204-2212.
10. Guo, W. L., Ngo, H. H., & Li, J. X. (2012). A mini-review on membrane fouling. Bioresource Technology, 122, 27-34.
Related Industry Knowledge
- Why are Titanium Electrodes Specifically Used for Ballast Water Management?
- How Do Titanium Electrodes Contribute to the Efficiency of Ballast Water Treatment?
- What Advantages do Titanium Electrodes Offer in Ballast Water Systems?
- How Do Titanium Electrodes Contribute to Ballast Water Treatment?
- What are the Advantages of Using Titanium Electrodes for Ballast Water Disinfection?
- Are There Any Safety Considerations When Using Titanium Electrodes for Ballast Water?
- What does a Ballast Water Titanium Electrode do?
- What are the Advantages of Using Titanium Electrodes for Ballast Water Management?
- Purifying the Seas: The Role of Titanium Electrodes in Ballast Water Treatment












