- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Ballast water titanium electrodes play a crucial role in the treatment of ballast water on ships. These electrodes are an essential component of electrochemical ballast water treatment systems, which are designed to eliminate harmful aquatic organisms and pathogens from ballast water before it's discharged into new environments. Titanium electrodes are specifically chosen for this application due to their excellent corrosion resistance, durability, and electrochemical properties. They function by generating disinfecting agents through electrolysis, effectively treating the ballast water and helping to prevent the spread of invasive species across different marine ecosystems.
How do titanium electrodes treat ballast water?
Titanium electrodes are at the heart of electrochemical ballast water treatment systems, employing a process known as electrolysis to disinfect ballast water. This method involves passing an electric current through the water, which triggers a series of chemical reactions at the electrode surfaces. The primary mechanism of action for titanium electrodes in ballast water treatment can be broken down into several key steps:
1. Generation of oxidizing agents: The electrolysis process also leads to the formation of powerful oxidizing agents, primarily hypochlorite (OCl-) and hypochlorous acid (HOCl). These compounds are highly effective in killing or inactivating a wide range of microorganisms, including bacteria, viruses, and other pathogens present in the ballast water.
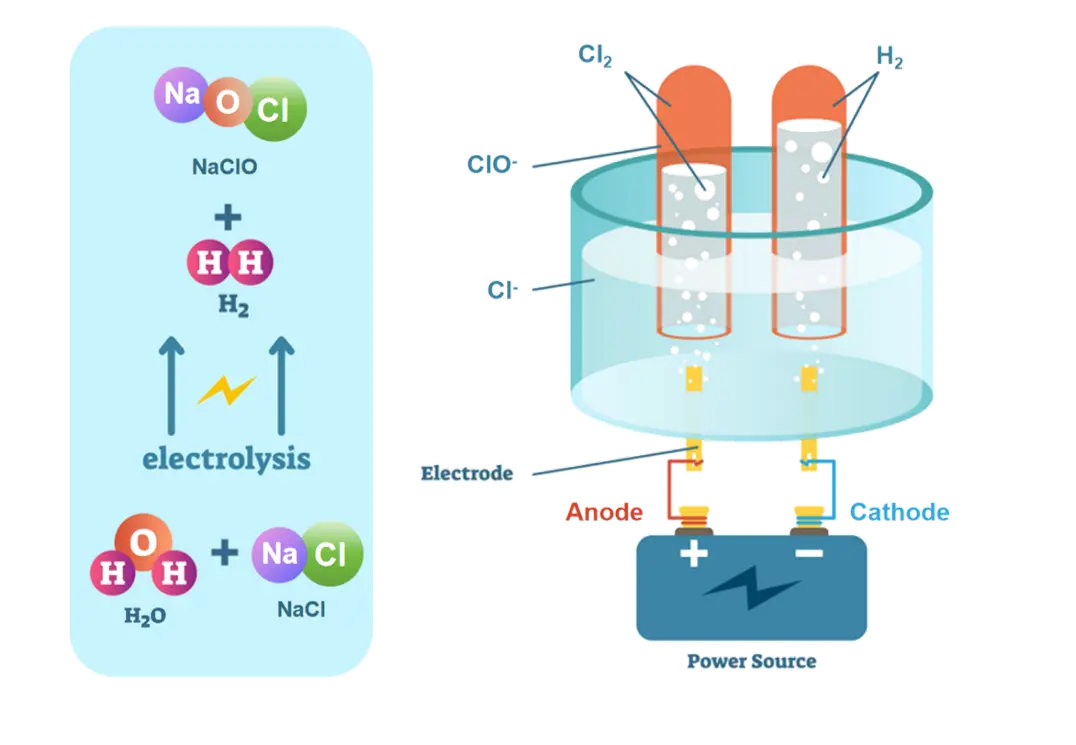
2. Direct oxidation: The titanium electrodes themselves can directly oxidize organic compounds and microorganisms in the water. This direct contact with the electrode surface can lead to the destruction of cell membranes and the inactivation of harmful organisms.
4. Hydroxyl radical formation: In some cases, the electrolysis process can also generate hydroxyl radicals (OH•), which are extremely potent oxidizing agents. These radicals can rapidly react with and destroy organic matter, including the cellular components of microorganisms.
5. pH alteration: The electrolysis process can cause localized changes in pH near the electrode surfaces. These pH shifts can create an inhospitable environment for many aquatic organisms, further contributing to their elimination.
6. Coagulation and flocculation: The electrochemical reactions at the titanium electrodes can also promote the coagulation and flocculation of suspended particles in the ballast water. This process helps to remove larger organisms and particulate matter, improving the overall water quality.
7. Continuous treatment: As the ballast water flows through the treatment system, it comes into contact with the titanium electrodes multiple times, ensuring thorough and consistent disinfection throughout the entire volume of water.
The use of titanium electrodes in this process is particularly advantageous due to their resistance to corrosion in the harsh marine environment. Titanium's ability to maintain its electrochemical properties over extended periods ensures the long-term effectiveness of the ballast water treatment system. Additionally, the dimensional stability of titanium electrodes helps to maintain consistent performance and energy efficiency throughout the treatment process.
What are the advantages of using titanium electrodes in ballast water treatment?
Titanium electrodes have become the material of choice for many ballast water treatment systems due to their numerous advantages over other electrode materials. These benefits contribute to the overall efficiency, reliability, and cost-effectiveness of the treatment process:
1. Corrosion resistance: One of the most significant advantages of titanium electrodes is their exceptional resistance to corrosion, even in the highly corrosive marine environment. This property ensures that the electrodes maintain their structural integrity and performance over extended periods, reducing the need for frequent replacements and minimizing maintenance costs.
2. Durability: Titanium electrodes are incredibly durable and can withstand the harsh conditions encountered in ballast water treatment systems. They are resistant to mechanical wear and tear, which is crucial given the constant flow of water and potential presence of abrasive particles.
3. High conductivity: Despite its corrosion resistance, titanium maintains excellent electrical conductivity when used as an electrode. This property ensures efficient energy transfer during the electrolysis process, contributing to the overall effectiveness of the treatment system.
4. Catalytic activity: Titanium electrodes can be coated with catalytic materials, such as mixed metal oxides, to enhance their electrochemical properties. These coatings can improve the efficiency of oxidant generation and increase the overall treatment effectiveness.
5. Long lifespan: Due to their corrosion resistance and durability, titanium electrodes have a significantly longer lifespan compared to other electrode materials. This longevity translates to reduced replacement costs and less frequent system downtime for maintenance.
6. Dimensional stability: Titanium electrodes maintain their shape and dimensions even after prolonged use in electrolytic processes. This stability ensures consistent performance and uniform current distribution, which is crucial for effective ballast water treatment.
7. Chemical inertness: Titanium is chemically inert in most environments, which means it does not react with or contaminate the ballast water being treated. This property is essential for maintaining water quality and preventing the introduction of unwanted substances into marine ecosystems.
8. Energy efficiency: The high conductivity and catalytic properties of titanium electrodes contribute to the overall energy efficiency of the ballast water treatment system. This efficiency can lead to reduced power consumption and lower operating costs for ship owners.
9. Versatility: Titanium electrodes can be used in various configurations and designs, allowing for flexibility in system design and optimization for different types of vessels and ballast water treatment requirements.
10. Compliance with regulations: The use of titanium electrodes in ballast water treatment systems helps shipowners comply with international regulations, such as the International Maritime Organization's Ballast Water Management Convention, by ensuring effective treatment of ballast water.
How effective are titanium electrodes in eliminating marine organisms?
The effectiveness of titanium electrodes in eliminating marine organisms from ballast water is a critical factor in their widespread adoption in treatment systems. Numerous studies and real-world applications have demonstrated the high efficacy of these electrodes in reducing the concentration of various aquatic species:
1. Broad-spectrum effectiveness: Titanium electrodes, when used in electrochemical ballast water treatment systems, have shown remarkable effectiveness against a wide range of marine organisms. This includes bacteria, viruses, phytoplankton, zooplankton, and even larger organisms such as fish larvae. The broad-spectrum action is primarily due to the generation of powerful oxidizing agents during the electrolysis process.
2. Rapid disinfection: The electrochemical reactions at the titanium electrode surfaces occur rapidly, leading to quick inactivation or destruction of microorganisms. This rapid action is crucial for treating large volumes of ballast water efficiently, especially during the short timeframes available during ballasting and de-ballasting operations.
3. Penetration of cell walls: The oxidizing agents produced by titanium electrodes, such as hypochlorite and hydroxyl radicals, can penetrate the cell walls of microorganisms. This penetration leads to the disruption of cellular functions and ultimately results in the death of the organisms.
4. Effectiveness against resistant species: Some marine organisms have developed resistance to traditional chemical treatments. However, the multiple mechanisms of action employed by titanium electrode systems, including direct oxidation and pH alteration, can overcome these resistance mechanisms, ensuring comprehensive treatment.
5. Reduction in viable organisms: Studies have shown that electrochemical systems using titanium electrodes can achieve significant reductions in the concentration of viable organisms in ballast water. Many systems report log reductions of 3 to 5 for various organism classes, meeting or exceeding the standards set by international regulations.
6. Minimal regrowth: The treatment process not only eliminates existing organisms but also helps prevent regrowth during ballast water storage. This is achieved through the residual disinfecting effects of the generated oxidants and the alteration of water chemistry.
7. Effectiveness in various water conditions: Titanium electrode systems have demonstrated effectiveness in treating ballast water with varying salinity, temperature, and turbidity levels. This versatility is crucial for ships operating in diverse marine environments.
8. Continuous monitoring and adjustment: Many advanced ballast water treatment systems incorporating titanium electrodes include real-time monitoring capabilities. These systems can adjust treatment parameters based on water quality and organism load, ensuring consistent effectiveness throughout the treatment process.
9. Compliance with discharge standards: The high efficacy of titanium electrode systems in eliminating marine organisms helps ships meet the stringent discharge standards set by international regulations, such as the IMO D-2 standard, which specifies maximum allowable concentrations for various organism size classes.
10. Long-term performance: The durability and corrosion resistance of titanium electrodes contribute to sustained effectiveness over time. Unlike some chemical treatment methods that may lose potency during storage, electrochemical systems can maintain their disinfection capabilities throughout the voyage.
In conclusion, ballast water titanium electrodes play a crucial role in protecting marine ecosystems by effectively treating ballast water and preventing the spread of invasive species. Their ability to generate powerful disinfecting agents through electrolysis, combined with their durability and corrosion resistance, makes them an ideal choice for shipboard ballast water treatment systems. As international regulations continue to tighten, the use of titanium electrodes in these systems will likely become even more widespread, contributing to the preservation of marine biodiversity and the health of our oceans.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
1. International Maritime Organization. (2004). International Convention for the Control and Management of Ships' Ballast Water and Sediments.
2. Tsolaki, E., & Diamadopoulos, E. (2010). Technologies for ballast water treatment: a review. Journal of Chemical Technology & Biotechnology, 85(1), 19-32.
3. Dang, K., Yin, P., Sun, P., Xiao, J., & Song, Y. (2018). Application of chemical disinfection and disinfection by-products (DBPs) in ballast water treatment: A review. Chemosphere, 218, 589-602.
4. Paolucci, E. M., Hernandez, M. R., Potapov, A., Lewis, M. A., & MacIsaac, H. J. (2015). Hybrid system increases efficiency of ballast water treatment. Journal of Applied Ecology, 52(2), 348-357.
5. Moreno-Andrés, J., Romero-Martínez, L., Acevedo-Merino, A., & Nebot, E. (2017). Effectiveness of a continuous flow-through electrolytic system for ballast water disinfection: Influence of the initial oxidant generated. Desalination, 429, 128-137.
6. Lacasa, E., Cañizares, P., Sáez, C., Fernández, F. J., & Rodrigo, M. A. (2013). Electrochemical phosphates removal using iron and aluminium electrodes. Chemical Engineering Journal, 228, 1-8.
7. Gollasch, S., David, M., Voigt, M., Dragsund, E., Hewitt, C., & Fukuyo, Y. (2007). Critical review of the IMO international convention on the management of ships' ballast water and sediments. Harmful algae, 6(4), 585-600.
8. Aliotta, J., Rogerson, A., Campbell, C. B., & Yonge, M. (2001). Ballast water treatment by electro-ionization. In Proceedings of the 1st International Ballast Water Treatment R&D Symposium, London, UK (pp. 61-69).
9. Leffler, C. E., Rogerson, A., & Paul, W. (2008). Electrochemical disinfection of ballast water: Laboratory investigations. In Proceedings of the Global R&D Forum on Compliance Monitoring and Enforcement: The Next R&D Challenge and Opportunity (pp. 21-23).
10. Nanayakkara, K. G. N., Alam, A. K. M. K., Zheng, Y. M., & Chen, J. P. (2012). A low-energy intensive electrochemical system for the eradication of Escherichia coli from ballast water: Process development, disinfection chemistry, and kinetics modeling. Marine Pollution Bulletin, 64(6), 1238-1245.
Related Industry Knowledge
- Why are Titanium Electrodes Specifically Used for Ballast Water Management?
- How Do Titanium Electrodes Contribute to the Efficiency of Ballast Water Treatment?
- What Advantages do Titanium Electrodes Offer in Ballast Water Systems?
- How Do Titanium Electrodes Contribute to Ballast Water Treatment?
- What are the Advantages of Using Titanium Electrodes for Ballast Water Disinfection?
- Are There Any Safety Considerations When Using Titanium Electrodes for Ballast Water?
- What are the Advantages of Using Titanium Electrodes for Ballast Water Management?
- Purifying the Seas: The Role of Titanium Electrodes in Ballast Water Treatment