- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
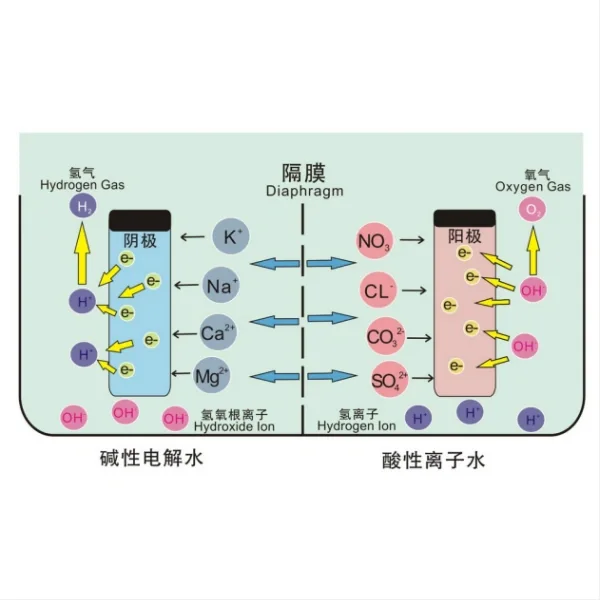
Ruthenium iridium coated titanium anodes are essential components in various electrochemical processes, known for their exceptional durability and efficiency. These anodes are manufactured through a sophisticated process that combines advanced materials science with precision engineering. The production involves carefully coating a titanium substrate with a mixture of ruthenium and iridium oxides, resulting in an electrode with superior catalytic properties and corrosion resistance. This blog post delves into the intricacies of manufacturing these high-performance anodes, exploring the key steps, technologies, and considerations involved in their production.
What are the Key Steps in the Manufacturing Process of Ruthenium Iridium Coated Titanium Anodes?
The manufacturing process of ruthenium iridium coated titanium anodes involves several critical steps, each contributing to the final product's quality and performance. The process begins with the preparation of the titanium substrate, which serves as the base for the electrode. This preparation typically involves cleaning and etching the titanium surface to ensure optimal adhesion of the coating.
The next crucial step is the preparation of the coating solution. This solution consists of ruthenium and iridium compounds, usually in the form of chlorides or other precursors, dissolved in an appropriate solvent. The precise composition of this solution is carefully controlled to achieve the desired ratio of ruthenium to iridium in the final coating.
Once the coating solution is prepared, it is applied to the titanium substrate. This application can be done through various methods, including thermal decomposition, electrodeposition, or spray pyrolysis. The thermal decomposition method is particularly common, where the coating solution is applied to the heated titanium substrate, causing the precursors to decompose and form a layer of mixed metal oxides.
After the initial coating application, the anode undergoes a series of thermal treatments. These treatments, often carried out at high temperatures in controlled atmospheres, are crucial for developing the desired crystal structure and properties of the coating. The thermal treatments help in forming a stable, adherent, and catalytically active layer of ruthenium and iridium oxides on the titanium surface.
The final steps in the manufacturing process involve quality control and testing. Each anode is thoroughly inspected to ensure uniform coating thickness, proper adhesion, and the absence of defects. Electrochemical tests are conducted to verify the anode's performance characteristics, including its catalytic activity and stability under operating conditions.
Throughout the manufacturing process, strict control of parameters such as temperature, atmosphere, and coating composition is maintained to ensure consistency and quality in the final product. The complexity of this process highlights the importance of specialized equipment and expertise in producing these high-performance anodes.
How Does the Coating Composition Affect the Performance of Ruthenium Iridium Anodes?
The composition of the coating on ruthenium iridium titanium anodes plays a crucial role in determining their performance characteristics. The ratio of ruthenium to iridium in the coating is a key factor that influences the anode's catalytic activity, durability, and overall efficiency in various electrochemical applications.
Ruthenium oxide is known for its excellent catalytic properties, particularly in chlorine evolution reactions. It exhibits high activity and low overpotential, making it efficient in processes such as chlor-alkali production. However, ruthenium oxide alone can be susceptible to dissolution under certain conditions, potentially limiting the anode's lifespan.
Iridium oxide, on the other hand, is renowned for its exceptional stability and resistance to corrosion. While it may not match ruthenium oxide in terms of catalytic activity for some reactions, its inclusion in the coating significantly enhances the durability of the anode. Iridium oxide is particularly valuable in applications involving oxygen evolution, where its stability under high anodic potentials is crucial.
By combining ruthenium and iridium oxides in the coating, manufacturers can create anodes that balance high catalytic activity with excellent durability. The synergistic effect of these two materials results in anodes that perform efficiently over extended periods, even in harsh electrochemical environments.
The optimal ratio of ruthenium to iridium in the coating depends on the specific application and operating conditions of the anode. For instance, anodes designed for chlorine production might have a higher proportion of ruthenium, while those intended for water treatment or oxygen evolution might contain more iridium. Some manufacturers offer customized coating compositions tailored to specific customer requirements and process conditions.
The coating composition also affects the anode's surface morphology and microstructure, which in turn influence its electrochemical properties. A well-designed coating will have a high surface area, providing numerous active sites for electrochemical reactions. This increased surface area can significantly enhance the anode's efficiency and reduce energy consumption in industrial processes.
Moreover, the coating composition can impact the anode's resistance to poisoning by impurities in the electrolyte. Certain additives or modifications to the coating formulation can improve the anode's tolerance to contaminants, extending its operational life in real-world applications.
Research in this field continues to explore new coating compositions and additives to further enhance the performance of ruthenium iridium coated titanium anodes. Recent developments include the incorporation of other noble metals or metal oxides to create multi-component coatings with improved characteristics for specific applications.
What Advancements Have Been Made in the Production Technology of These Anodes?
The production technology for ruthenium iridium coated titanium anodes has seen significant advancements in recent years, driven by the increasing demand for more efficient and durable electrodes in various industries. These innovations have focused on improving coating methods, enhancing material utilization, and developing more sophisticated quality control techniques.
One notable advancement is in the area of coating application methods. Traditional thermal decomposition techniques have been refined to achieve more uniform and adherent coatings. Advanced spray techniques, such as plasma spraying and flame spraying, have been adapted for applying ruthenium iridium coatings, allowing for better control over coating thickness and composition.
Electrodeposition methods have also seen improvements, with the development of pulse plating techniques that can produce coatings with enhanced microstructure and performance characteristics. These methods allow for better control over the coating's porosity and surface area, crucial factors in the anode's catalytic activity.
Nanotechnology has played a significant role in recent advancements. The development of nanostructured coatings has led to anodes with dramatically increased surface areas and improved catalytic properties. These nanostructured coatings can be achieved through various methods, including sol-gel processes and electrospinning techniques.
Another area of advancement is in the precursor materials used for coating. New precursor formulations have been developed that allow for better control over the coating composition and properties. These include organometallic precursors that decompose at lower temperatures, reducing energy consumption in the manufacturing process and minimizing thermal stress on the titanium substrate.
Automation and process control have also seen significant improvements. Advanced monitoring systems and real-time process control allow for more consistent product quality and reduced manufacturing costs. Computer-controlled coating systems can adjust parameters dynamically during the coating process, ensuring optimal results.
Quality control and characterization techniques have advanced as well. High-resolution microscopy techniques, such as scanning electron microscopy (SEM) and atomic force microscopy (AFM), are now routinely used to examine coating morphology and structure at the nanoscale. Advanced electrochemical testing methods provide more detailed information about the anode's performance characteristics under various conditions.
Environmental considerations have also driven innovations in the production process. Manufacturers are developing more sustainable production methods that reduce waste and energy consumption. This includes the development of closed-loop systems for recovering and recycling precious metals from the coating process.
Research into alternative materials is ongoing, with efforts to develop coatings that maintain high performance while reducing reliance on scarce and expensive noble metals. This includes exploring mixed-metal oxide coatings that incorporate more abundant elements while maintaining the desired electrochemical properties.
The integration of computational modeling and simulation in the design and optimization of coating compositions and processes represents another frontier in anode production technology. These tools allow manufacturers to predict coating performance and optimize production parameters more efficiently, reducing the time and cost associated with empirical testing.
These advancements in production technology have collectively contributed to the development of ruthenium iridium coated titanium anodes with improved performance, longer lifespans, and better cost-effectiveness. As research continues and new technologies emerge, further improvements in anode manufacturing and performance are anticipated, driving innovation in various electrochemical industries.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References
1. Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
2. Chen, R., Trieu, V., Zeradjanin, A. R., & Natter, H. (2020). Electrodeposited Ir-Ru mixed oxides as highly efficient and stable electrodes for the oxygen evolution reaction. ACS Catalysis, 10(14), 8013-8020.
3. Xu, L., Xiao, Y., van Sandwijk, A., Xu, Q., & Yang, Y. (2015). Production of ruthenium and iridium coated titanium electrodes by thermal decomposition. Materials, 8(4), 1289-1301.
4. Malpass, G. R. P., Neves, R. S., & Motheo, A. J. (2006). A comparative study of commercial and laboratory-made Ti/Ru0.3Ti0.7O2 DSA® electrodes: "In situ" and "ex situ" surface characterisation and organic oxidation activity. Electrochimica Acta, 52(3), 936-944.
5. Krstajić, N., Trasatti, S., & Atanasoski, R. (1984). Oxygen evolution on a Ru+Ir/Ti catalyst. Journal of Applied Electrochemistry, 14(5), 611-618.
6. Comninellis, C., & Vercesi, G. P. (1991). Characterization of DSA®-type oxygen evolving electrodes: Choice of a coating. Journal of Applied Electrochemistry, 21(4), 335-345.
7. De Pauli, C. P., & Trasatti, S. (2002). Electrochemical surface characterization of IrO2 + SnO2 mixed oxide electrocatalysts. Journal of Electroanalytical Chemistry, 538, 145-151.
8. Marshall, A. T., & Haverkamp, R. G. (2010). Electrocatalytic activity of IrO2–RuO2 supported on Sb-doped SnO2 nanoparticles. Electrochimica Acta, 55(6), 1978-1984.
9. Ardizzone, S., Bianchi, C. L., Borgese, L., Cappelletti, G., Locatelli, C., Minguzzi, A., ... & Vertova, A. (2008). High surface area Nb-doped TiO2 as cathode for oxidative destruction of organics. Journal of Applied Electrochemistry, 38(10), 1411-1419.
10. Chen, S., Zheng, Y., Wang, S., & Chen, X. (2011). Ti/RuO2–Sb2O5–SnO2 electrodes for chlorine evolution from seawater. Chemical Engineering Journal, 172(1), 47-51.
Related Industry Knowledge
- Can Copper Be Used as an Electrode?
- How do I Choose a MMO Tubular Titanium Anode?
- Can Titanium Electrodes Be Used with Saltwater Chlorinators?
- Can Titanium Electrodes be Used for Large-Scale Water Treatment?
- How Do Titanium Anodes Enhance Zinc Electrowinning Processes?
- Electrochemical Essentials: The Comprehensive Guide to Anode Plates
- How Does a DSA Anode Revolutionize Electrochemical Processes?
- What Industries Rely on DSA Anodes for Electrochemical Processes?