- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Can Copper Be Used as an Electrode?
2024-07-10 10:24:16
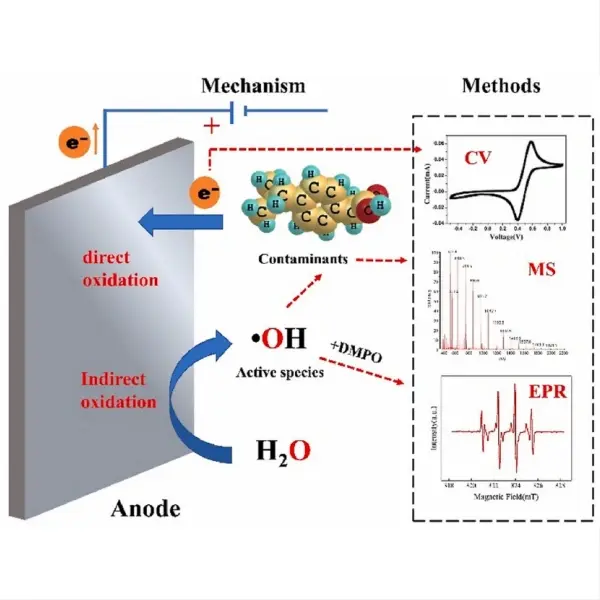
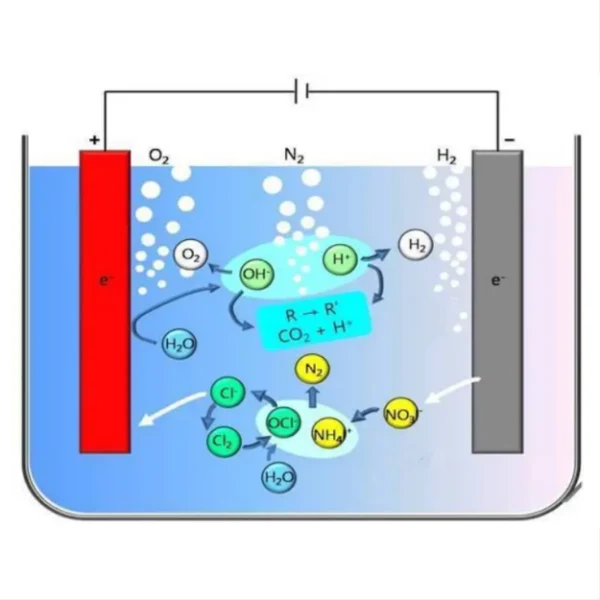
Copper is indeed a popular choice for electrodes in various applications due to its excellent electrical conductivity, availability, and relatively low cost. As a highly conductive metal, copper can efficiently transfer electrons, making it suitable for use in electrochemical processes, electrical systems, and energy storage devices. Its malleability and ductility also allow for easy shaping and fabrication into various electrode forms. While copper electrodes have their advantages, they may not be ideal for all situations, particularly in highly corrosive environments or where specific chemical reactions are required. Let's explore some key aspects of copper electrodes and their relationship with titanium electrodes.
How does copper compare to titanium as an electrode material?
Copper and titanium are both widely used electrode materials, each with its own set of advantages and limitations. Copper is known for its exceptional electrical conductivity, which is about four times higher than that of titanium. This property makes copper an excellent choice for applications where minimal electrical resistance is crucial, such as in power transmission or high-current electrochemical processes.
However, titanium has some distinct advantages over copper in certain scenarios. One of the most significant benefits of titanium is its superior corrosion resistance. While copper can corrode in acidic or oxidizing environments, titanium forms a stable, protective oxide layer on its surface, making it highly resistant to corrosion in a wide range of chemical environments. This property is particularly valuable in harsh industrial settings or marine applications where electrodes are exposed to corrosive substances.
Titanium also has a higher melting point than copper (1668°C vs. 1084°C), which makes it more suitable for high-temperature applications. Additionally, titanium has a lower density than copper, making it a lighter option for weight-sensitive applications.
In terms of mechanical properties, titanium generally offers higher strength and hardness compared to copper. This can be advantageous in applications where the electrode must withstand mechanical stress or wear.
The choice between copper and titanium as an electrode material often depends on the specific requirements of the application. For instance, in electroplating processes where high conductivity is crucial, copper might be preferred. On the other hand, in corrosive environments like seawater electrolysis, titanium electrodes are often the better choice due to their superior corrosion resistance.
It's worth noting that in some cases, the advantages of both materials can be combined. For example, copper-clad titanium electrodes are sometimes used to leverage the high conductivity of copper with the corrosion resistance of titanium. This composite material can offer a balance of properties that neither metal alone can provide.
When considering cost, copper is generally less expensive than titanium, which can be a significant factor in large-scale applications. However, the longer lifespan of titanium electrodes in corrosive environments may offset the initial higher cost in some cases.
In summary, while copper excels in electrical conductivity and cost-effectiveness, titanium offers superior corrosion resistance and strength. The choice between the two depends on the specific requirements of the application, including the operating environment, conductivity needs, durability requirements, and budget constraints.
What are the advantages of electrodepositing copper on titanium electrodes?
Electrodepositing copper on titanium electrodes is a technique that combines the beneficial properties of both metals, creating a composite electrode with unique characteristics. This process involves depositing a layer of copper onto a titanium substrate through electroplating. The resulting electrode can offer several advantages over using either copper or titanium alone.
One of the primary benefits of electrodepositing copper on titanium electrodes is the enhancement of electrical conductivity. While titanium is valued for its corrosion resistance and strength, its electrical conductivity is relatively low compared to copper. By adding a layer of copper to the titanium surface, the overall conductivity of the electrode can be significantly improved. This is particularly useful in applications where the corrosion resistance of titanium is needed, but higher conductivity is also required.
The copper layer on the titanium substrate can also improve the electrode's performance in certain electrochemical reactions. Copper is known to be an effective catalyst for various reactions, including the reduction of carbon dioxide to hydrocarbons. By combining copper's catalytic properties with titanium's stability, these composite electrodes can enhance reaction rates and efficiencies in specific electrochemical processes.
Another advantage of this composite structure is the potential for cost reduction. Pure titanium electrodes can be expensive, especially for large-scale applications. By using a titanium base with a copper coating, it's possible to reduce the overall amount of titanium used while still maintaining its beneficial properties. The copper layer provides the necessary conductivity, while the titanium substrate ensures structural integrity and corrosion resistance.
The durability of the electrode can also be enhanced through this electrodeposition process. The titanium substrate provides excellent mechanical strength and resistance to wear, while the copper layer can be easily renewed if it becomes degraded over time. This can lead to longer-lasting electrodes that require less frequent replacement, potentially reducing maintenance costs and downtime in industrial applications.
Furthermore, the electrodeposition process allows for precise control over the thickness and properties of the copper layer. This flexibility enables the customization of electrodes for specific applications, optimizing the balance between conductivity, catalytic activity, and corrosion resistance as needed.
In some cases, the copper-titanium interface can exhibit unique properties that neither metal possesses individually. For instance, the junction between the two metals can create localized electric fields or strain effects that may be beneficial for certain electrochemical reactions or sensing applications.
It's important to note that while electrodepositing copper on titanium offers many advantages, there are also challenges to consider. Ensuring strong adhesion between the copper layer and the titanium substrate is crucial to prevent delamination during use. Additionally, the electrodeposition process must be carefully controlled to achieve uniform coverage and desired copper layer properties.
In conclusion, electrodepositing copper on titanium electrodes can create a versatile composite material that combines the strengths of both metals. This technique offers improved conductivity, enhanced catalytic activity, potential cost savings, and customizable properties. As research in this area continues, we can expect to see further refinements and new applications for these composite electrodes in various fields, from energy storage and conversion to environmental remediation and industrial processes.
How does the performance of copper-electrodeposited titanium electrodes compare to other electrode materials?
The performance of copper-electrodeposited titanium electrodes can be evaluated in comparison to other common electrode materials used in various applications. This comparison is essential for understanding the unique advantages and potential limitations of these composite electrodes in different contexts.
When compared to pure copper electrodes, copper-electrodeposited titanium electrodes generally offer improved durability and corrosion resistance. While pure copper electrodes excel in conductivity, they can be susceptible to corrosion in acidic or oxidizing environments. The titanium substrate in the composite electrode provides a protective base that can withstand harsh conditions more effectively. This makes copper-electrodeposited titanium electrodes particularly advantageous in applications where the electrode may be exposed to corrosive substances or extreme pH levels.
In comparison to pure titanium electrodes, the copper-electrodeposited version typically demonstrates superior electrical conductivity. This enhanced conductivity can lead to improved energy efficiency in electrochemical processes, as it reduces the electrical resistance and associated energy losses. The copper layer also introduces catalytic properties that pure titanium may lack, potentially improving reaction rates for certain electrochemical processes.
When considering noble metal electrodes like platinum or gold, copper-electrodeposited titanium electrodes often present a more cost-effective alternative. While noble metals offer excellent catalytic properties and corrosion resistance, their high cost can be prohibitive for large-scale applications. Copper-electrodeposited titanium electrodes can provide a balance of performance and affordability, making them attractive for industrial use.
In the context of carbon-based electrodes, such as graphite or glassy carbon, copper-electrodeposited titanium electrodes generally offer higher conductivity and mechanical strength. However, carbon electrodes may have advantages in terms of chemical inertness and affordability for certain applications.
The performance of copper-electrodeposited titanium electrodes in specific applications can vary depending on factors such as the thickness and quality of the copper layer, the surface preparation of the titanium substrate, and the particular electrochemical environment. For instance, in chlor-alkali production, these composite electrodes have shown promising results, offering good stability and efficiency compared to traditional electrode materials.
In electrochemical sensing applications, copper-electrodeposited titanium electrodes have demonstrated enhanced sensitivity for certain analytes compared to bare titanium or other common electrode materials. The copper layer can provide active sites for specific reactions, while the titanium substrate ensures stability and reusability of the sensor.
For energy storage applications, such as in supercapacitors or batteries, copper-electrodeposited titanium electrodes have shown potential for improving charge storage capacity and cycle life. The combination of copper's high conductivity with titanium's stability can lead to electrodes that maintain performance over many charge-discharge cycles.
It's important to note that the performance of copper-electrodeposited titanium electrodes can be further enhanced through additional modifications. For example, incorporating nanostructures or additional catalytic materials into the copper layer can tailor the electrode's properties for specific reactions or applications.
While copper-electrodeposited titanium electrodes offer many advantages, they may not always be the optimal choice for every application. Factors such as the specific electrochemical reaction, operating conditions, scale of the application, and cost considerations all play a role in determining the most suitable electrode material.
In conclusion, copper-electrodeposited titanium electrodes present a versatile option that can compete favorably with other electrode materials in many applications. Their combination of conductivity, durability, and cost-effectiveness makes them particularly well-suited for scenarios requiring a balance of these properties. As research in this field continues, we can expect further improvements in the performance and applicability of these composite electrodes, potentially expanding their use across various industries and technological domains.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Walsh, F. C., & Ponce de León, C. (2014). A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying technology. Transactions of the IMF, 92(2), 83-98.
2. Kasem, K. K., & Jones, S. (2008). Platinum as a reference electrode in electrochemical measurements. Platinum Metals Review, 52(2), 100-106.
3. Chen, S., Duan, J., Jaroniec, M., & Qiao, S. Z. (2013). Three-dimensional N-doped graphene hydrogel/NiCo double hydroxide electrocatalysts for highly efficient oxygen evolution. Angewandte Chemie International Edition, 52(51), 13567-13570.
4. Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
5. Mehta, V., & Cooper, J. S. (2003). Review and analysis of PEM fuel cell design and manufacturing. Journal of Power Sources, 114(1), 32-53.
6. Pletcher, D., & Walsh, F. C. (1990). Industrial electrochemistry. Springer Science & Business Media.
7. Bard, A. J., & Faulkner, L. R. (2001). Electrochemical methods: Fundamentals and applications. New York: Wiley.
8. Comninellis, C., & Chen, G. (Eds.). (2010). Electrochemistry for the environment. Springer Science & Business Media.
9. Schlesinger, M., & Paunovic, M. (Eds.). (2011). Modern electroplating (Vol. 55). John Wiley & Sons.
10. Sequeira, C. A. C., & Santos, D. M. F. (Eds.). (2017). Electrochemical power sources: Batteries, fuel cells, and supercapacitors. John Wiley & Sons.