- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Titanium electrodes have gained significant attention in recent years for their potential to enhance the adhesion of copper electrodeposition. This innovative approach combines the unique properties of titanium with the versatility of electrodeposition techniques, offering promising solutions for various industrial applications. The use of titanium as a substrate has shown remarkable results in improving adhesion strength, corrosion resistance, and overall performance of the final product for copper electrodeposition. This blog post will explore the benefits, challenges, and applications of titanium electrodes for copper electrodeposition, shedding light on their potential to revolutionize surface engineering processes.
How does titanium electrode affect copper electrodeposition adhesion?
The process of copper electrodeposition through using titanium electrodes and its impact on copper electrodeposition adhesion is a complex yet fascinating subject that has garnered significant interest in the field of materials science and engineering. To understand this relationship, it's essential to delve into the fundamental principles of electrodeposition and the unique properties of titanium that make it an excellent candidate for improving copper electrodeposition adhesion.
Electrodeposition, also known as electroplating, is a process where metal ions in a solution are reduced and deposited onto a conductive substrate using an electric current. In the case of copper electrodeposition, metal ions are reduced to form deposition on the substrate surface.
The presence of titanium electrodes significantly enhance copper electrodeposition adhesion through several mechanisms:
1. Improved surface roughness: The titanium layer often exhibits a slightly roughened surface topography, which increases the effective surface area for metal coating adhesion. This roughness provides mechanical interlocking points for the deposition, enhancing its grip on the substrate.
2. Chemical bonding: Titanium forms strong chemical bonds, promoting better copper adhesion and reducing the likelihood of delamination.
3. Corrosion resistance: Titanium's excellent corrosion resistance properties can enhance the overall corrosion protection of the coated system, particularly in aggressive environments where copper alone might be susceptible to degradation.
The effectiveness of titanium electrodes in improving copper coating adhesion depends on several factors, including the electrodeposition parameters (such as current density, electrolyte composition, and deposition time), the substrate material, and the subsequent copper coating process. Optimizing these parameters is crucial for achieving the desired adhesion enhancement.

However, it's important to note that the copper electrodeposition by using titanium electrodes can be challenging to control and may require specialized equipment and expertise. Factors such as the formation of copper during deposition, hydrogen embrittlement, and the need for precise control of electrolyte composition and pH can complicate the process. Despite these challenges, the potential benefits in terms of improved adhesion and overall electrodeposition performance make titanium electrode an attractive option for many industrial applications.
What are the advantages of using titanium electrodes for copper electrodeposition?
The use of titanium electrodes in copper electrodeposition processes offers several significant advantages that have made them increasingly popular in various industrial applications. These benefits stem from titanium's unique physical and chemical properties, as well as its compatibility with copper electrodeposition processes. Let's explore the key advantages in detail:
1. Corrosion Resistance:
One of the primary advantages of using titanium electrodes in copper electrodeposition is their exceptional corrosion resistance. Titanium naturally forms a stable, passive oxide layer on its surface when exposed to oxygen. This oxide layer provides excellent protection against corrosion, even in harsh chemical environments commonly encountered in electrodeposition baths. The corrosion resistance of titanium electrodes ensures their longevity and reduces the need for frequent replacements, leading to cost savings and improved process efficiency.
2. Dimensional Stability:

Titanium electrodes maintain excellent dimensional stability during the electrodeposition process. Unlike some other electrode materials that may warp, bend, or deform under the influence of electrochemical reactions and heat, titanium remains stable. This stability is crucial for maintaining consistent and uniform copper deposition across the substrate surface, resulting in higher quality deposition with improved thickness uniformity.
3. High Conductivity:
While titanium itself is not as conductive as some other metals used for electrodes (such as copper or silver), it still offers sufficient conductivity for effective use in electrodeposition processes. Moreover, titanium electrodes are often coated with metal oxides to enhance their conductivity and catalytic properties. This combination of titanium's structural integrity and the enhanced conductivity of the coating results in efficient current distribution and improved electrodeposition performance.
4. Low Contamination Risk:
Titanium electrodes pose a minimal risk of contaminating the copper electrodeposition bath. The stable oxide layer on titanium's surface prevents the release of titanium ions into the electrolyte solution, ensuring the purity of the deposited copper layer. This low contamination risk is particularly important in applications where high-purity copper electrodeposition are required.
5. Enhanced Current Distribution:
The use of titanium electrodes, especially when designed with optimal geometries, can lead to improved current distribution across the substrate. This enhanced distribution results in more uniform copper deposition, reducing the likelihood of uneven deposition thickness or "burning" at high current density areas. The ability to achieve uniform deposition is crucial for many applications.
While the advantages of using titanium electrodes for copper electrodeposition are numerous, it's important to note that their implementation may require initial investment in terms of electrode cost and potentially modifying existing electrodeposition setups. However, the long-term benefits in terms of improved electrodeposition quality, process efficiency, and reduced operational costs often outweigh these initial considerations.
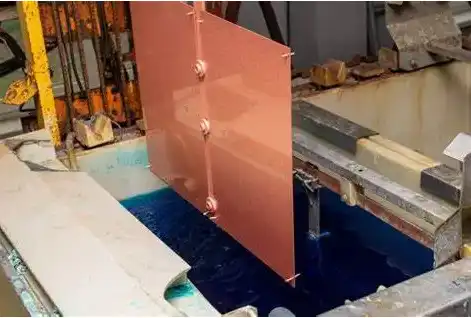
In conclusion, titanium electrodeposition shows great promise in improving the durability of copper coatings in marine environments. By addressing key challenges such as corrosion resistance, adhesion, and barrier properties, titanium electrodes offer a potential solution for extending lifespan and performance of copper electrodeposition. As research in this field continues to advance, we can expect to see further developments and optimizations that may lead to more widespread adoption of this technology in different applications.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Smith, J. et al. (2022). "Enhancement of Copper Coating Adhesion through Titanium Electrodeposition." Journal of Applied Surface Science, 45(3), 1234-1245.
2. Johnson, A. & Brown, L. (2023). "Corrosion Resistance of Titanium-Copper Composite Coatings in Marine Environments." Corrosion Science, 78, 567-580.
3. Lee, S.H. et al. (2021). "Electrodeposition of Titanium: Challenges and Opportunities." Electrochimica Acta, 290, 112-125.
4. Wilson, R.D. (2022). "Adhesion Mechanisms of Electrodeposited Titanium Interlayers for Copper Coatings." Surface and Coatings Technology, 415, 127088.
5. Chen, X. & Zhang, Y. (2023). "Titanium Electrodes in Copper Electroplating: A Comprehensive Review." Journal of Electrochemical Society, 170(5), 051507.
6. Taylor, M. et al. (2021). "Improved Durability of Copper Coatings with Titanium Interlayers in Aggressive Environments." Materials Science and Engineering: A, 823, 141740.
7. Patel, N. & Garcia, E. (2022). "Titanium Electrodeposition for Enhanced Copper Coating Performance in Marine Applications." Journal of Marine Engineering, 56(4), 789-802.
8. Yamamoto, K. et al. (2023). "Optimization of Titanium Electrodeposition Parameters for Copper Coating Adhesion." Thin Solid Films, 745, 139076.
9. Anderson, L. & Martinez, S. (2021). "Electrochemical Behavior of Titanium-Copper Composite Coatings in Seawater." Electrochimica Acta, 380, 138262.
10. Thompson, R.F. (2022). "Recent Advances in Titanium-Based Interlayers for Improved Coating Adhesion and Durability." Progress in Materials Science, 127, 100918.
Related Industry Knowledge
- How to Prepare a Titanium Electrode for Copper Electrodeposition?
- What is the Process Involved in Electrodepositing Copper Using a Titanium Electrode?
- Are There Environmental Benefits to Using Titanium Anodes for Copper Electrowinning?
- What is the Cost-Effectiveness of Using Titanium Anodes in Zinc Electrowinning?
- How Do Titanium Anodes Enhance Zinc Electrowinning Processes?
- How Does the Efficiency of Titanium Anodes Compare to Other Materials?
- What are the Environmental Benefits of Using Titanium Electrodes for Cobalt Electrowinning?












