- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
What is the Process Involved in Electrodepositing Copper Using a Titanium Electrode?
Electrodeposition of copper using titanium electrodes is a fascinating process in the world of electrochemistry and materials science. This technique involves the use of an electric current to deposit a layer of copper onto a titanium substrate, combining the excellent properties of both metals. The process has gained significant attention in various industries due to its ability to create high-quality coatings with enhanced durability and conductivity. In this blog post, we'll explore the intricacies of this electrodeposition process, its applications, and the key factors that influence its success.
How does the electrodeposition of copper on titanium work?
The electrodeposition of copper on titanium is a complex electrochemical process that involves several steps and carefully controlled conditions. To understand this process, we need to delve into the fundamentals of electrochemistry and the specific characteristics of both copper and titanium.
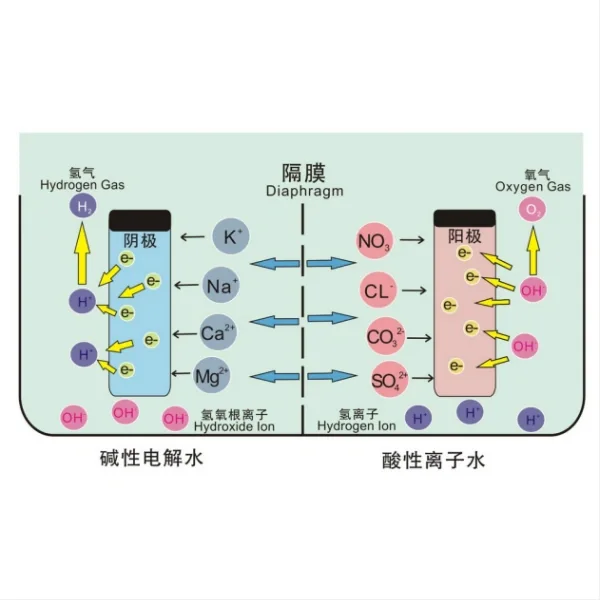
At its core, the electrodeposition process involves the reduction of copper ions from an electrolyte solution onto a titanium cathode. The titanium electrode serves as the substrate onto which the copper will be deposited. This process takes place in an electrochemical cell, which typically consists of the following components:
1. Anode: Usually made of copper, which serves as the source of copper ions.
2. Cathode: The titanium electrode onto which copper will be deposited.
3. Electrolyte: An aqueous solution containing copper ions, typically copper sulfate (CuSO4) dissolved in water, along with other additives to enhance conductivity and control the deposition process.
4. Power source: A direct current (DC) power supply that provides the necessary electrical energy for the reaction.
The electrodeposition process begins when an electric current is applied to the system. This causes the copper anode to oxidize, releasing copper ions (Cu2+) into the electrolyte solution. Simultaneously, at the titanium cathode, copper ions from the electrolyte are reduced and deposited as metallic copper (Cu) onto the titanium surface.
The overall reaction can be summarized as follows:
At the anode: Cu → Cu2+ + 2e-
At the cathode: Cu2+ + 2e- → Cu
Several factors influence the quality and characteristics of the deposited copper layer:
1. Current density: The amount of current applied per unit area of the electrode surface affects the rate of deposition and the properties of the resulting copper layer.
2. Electrolyte composition: The concentration of copper ions, pH, and presence of additives in the electrolyte solution can significantly impact the deposition process and the properties of the deposited copper.
3. Temperature: The temperature of the electrolyte solution affects the rate of deposition and the morphology of the deposited copper.
4. Agitation: Stirring or flowing the electrolyte solution can help maintain a uniform concentration of copper ions near the cathode surface, leading to more even deposition.
5. Substrate preparation: The condition of the titanium surface, including its cleanliness and roughness, can affect the adhesion and quality of the deposited copper layer.
6. Deposition time: The duration of the electrodeposition process determines the thickness of the copper layer.
One of the challenges in electrodepositing copper on titanium is ensuring good adhesion between the two metals. Titanium has a natural oxide layer on its surface, which can interfere with the deposition process. To overcome this, the titanium surface is often pretreated through methods such as mechanical abrasion, chemical etching, or electrochemical activation to remove the oxide layer and create a more receptive surface for copper deposition.
The electrodeposition of copper on titanium offers several advantages. The resulting composite material combines the excellent corrosion resistance and high strength-to-weight ratio of titanium with the superior electrical and thermal conductivity of copper. This makes it particularly useful in applications where these properties are crucial, such as in the electronics industry, aerospace sector, and energy storage devices.
What are the key factors affecting the quality of electrodeposited copper on titanium?
The quality of electrodeposited copper on titanium is crucial for its performance in various applications. Several key factors influence the deposition process and the resulting copper layer's properties. Understanding and controlling these factors is essential for achieving high-quality coatings that meet specific requirements.
1. Current density:
The current density, which is the amount of electrical current flowing per unit area of the electrode surface, is one of the most critical parameters in electrodeposition. It directly affects the rate of copper deposition and the morphology of the deposited layer.
- Low current density: Generally results in slower deposition rates and can lead to a more compact and fine-grained copper structure. However, if the current density is too low, it may result in poor adhesion or incomplete coverage.
- High current density: Increases the deposition rate but can lead to rougher, less uniform deposits. Extremely high current densities may cause "burning" or dendritic growth of the copper, resulting in a powdery or spongy deposit.
The optimal current density depends on the specific application and desired properties of the copper coating. It often requires experimentation and fine-tuning to achieve the best results.
2. Electrolyte composition:
The composition of the electrolyte solution plays a crucial role in the electrodeposition process. The main components of the electrolyte include:
- Copper salt: Typically copper sulfate (CuSO4), which provides the source of copper ions.
- Sulfuric acid: Added to increase conductivity and maintain the pH of the solution.
- Additives: Various organic and inorganic compounds that can be added to modify the properties of the deposited copper.
The concentration of copper ions in the electrolyte affects the deposition rate and the quality of the deposit. A higher concentration generally allows for higher current densities and faster deposition rates. However, it's essential to maintain the copper ion concentration within an optimal range to ensure consistent deposition quality.
Additives in the electrolyte can significantly influence the properties of the deposited copper. Common additives include:
- Leveling agents: Help to produce smoother, more uniform deposits by preferentially inhibiting deposition on protrusions.
- Brighteners: Promote the formation of smaller grain sizes, resulting in a brighter, more reflective copper surface.
- Stress reducers: Help to minimize internal stresses in the deposited copper layer, reducing the risk of cracking or peeling.
- Wetting agents: Improve the wetting of the titanium surface, enhancing adhesion and coverage.
3. pH of the electrolyte:
The pH of the electrolyte solution affects the efficiency of the deposition process and the properties of the deposited copper. For copper electrodeposition, the optimal pH range is typically between 0.5 and 2.0.
- Lower pH (more acidic): Tends to produce finer-grained deposits but may increase hydrogen evolution, which can lead to embrittlement of the deposit.
- Higher pH: Can result in coarser-grained deposits and may lead to the formation of basic copper salts, which can interfere with the deposition process.
Careful control of the pH is necessary to maintain consistent deposition quality and prevent unwanted side reactions.
4. Temperature:
The temperature of the electrolyte solution affects various aspects of the electrodeposition process:
- Deposition rate: Higher temperatures generally increase the deposition rate due to increased ion mobility and reaction kinetics.
- Grain size: Elevated temperatures often lead to larger grain sizes in the deposited copper.
- Stress: The internal stress in the deposited copper layer can be influenced by temperature, with higher temperatures typically resulting in lower stress.
- Additive effectiveness: The performance of certain additives in the electrolyte may be temperature-dependent.
The optimal temperature range for copper electrodeposition is typically between 20°C and 30°C, although this can vary depending on the specific application and desired properties.
5. Agitation:
Proper agitation of the electrolyte solution is crucial for achieving uniform and high-quality copper deposits. Agitation serves several purposes:
- Maintains a uniform concentration of copper ions near the cathode surface, preventing depletion.
- Helps to remove hydrogen bubbles that may form on the cathode surface, which can interfere with the deposition process.
- Promotes mass transfer of ions, improving the overall efficiency of the process.
Agitation can be achieved through various methods, including mechanical stirring, air sparging, or solution flow. The optimal level of agitation depends on factors such as the geometry of the electrodeposition setup, the current density, and the desired properties of the copper deposit.
By carefully controlling and optimizing these key factors, it's possible to achieve high-quality electrodeposited copper coatings on titanium substrates with the desired properties for specific applications. This may involve a process of experimentation and fine-tuning to determine the optimal parameters for a given set of requirements.
What are the main applications of copper-electrodeposited titanium electrodes?
Copper-electrodeposited titanium electrodes have found a wide range of applications across various industries due to their unique combination of properties. These composite materials harness the strengths of both copper and titanium, offering excellent electrical and thermal conductivity along with high strength, light weight, and corrosion resistance. Let's explore some of the main applications of these versatile electrodes:
1. Electronics and Semiconductor Industry:
In the rapidly evolving world of electronics, copper-electrodeposited titanium electrodes play a crucial role in several applications:
- Printed Circuit Boards (PCBs): The high conductivity of copper combined with the strength and light weight of titanium makes these electrodes ideal for high-performance PCBs, especially in applications where weight is a critical factor, such as in aerospace or portable electronics.
- Semiconductor Manufacturing: These electrodes are used in various stages of semiconductor production, including in electroplating processes for creating interconnects and in plasma etching equipment where their corrosion resistance is valuable.
- Electromagnetic Shielding: The excellent conductivity of copper combined with the strength of titanium makes these electrodes useful for creating effective electromagnetic shields in electronic devices.
2. Energy Storage and Conversion:
The unique properties of copper-electrodeposited titanium electrodes make them valuable in various energy-related applications:
- Batteries: These electrodes are used in advanced battery designs, particularly in high-performance lithium-ion batteries. The copper layer provides excellent conductivity while the titanium substrate offers strength and corrosion resistance.
- Fuel Cells: In certain types of fuel cells, particularly solid oxide fuel cells, these electrodes can be used as interconnects or current collectors, benefiting from their high conductivity and resistance to high-temperature corrosion.
- Supercapacitors: The high surface area and conductivity of these electrodes make them suitable for use in supercapacitors, where rapid charge and discharge capabilities are crucial.
3. Aerospace and Aviation:
The aerospace industry benefits from the light weight and high strength of titanium combined with the conductivity of copper:
- Aircraft Wiring: Copper-electrodeposited titanium wires offer a lightweight alternative to traditional copper wiring in aircraft, helping to reduce overall weight without compromising on electrical performance.
- Satellite Components: In satellite applications, where every gram matters, these electrodes provide an excellent balance of conductivity and weight savings.
- Lightning Protection: The high conductivity of the copper layer combined with the strength of titanium makes these materials useful in aircraft lightning protection systems.
4. Corrosion-Resistant Applications:
The corrosion resistance of titanium, enhanced by the protective copper layer, makes these electrodes valuable in harsh environments:
- Marine Applications: In seawater environments, these electrodes can be used for various purposes, including in desalination plants, offshore structures, and marine research equipment.
- Chemical Processing: In the chemical industry, where corrosion resistance is crucial, these electrodes find use in electrochemical processes and as components in processing equipment.
5. Medical Devices:
The biocompatibility of titanium, combined with the antimicrobial properties of copper, makes these electrodes interesting for certain medical applications:
- Implantable Devices: While further research is needed, there is potential for using these materials in certain implantable medical devices where both conductivity and biocompatibility are required.
- Medical Imaging Equipment: In MRI machines and other medical imaging devices, these electrodes can be used in components where high conductivity and low magnetic susceptibility are needed.
6. Telecommunications:
The telecommunications industry benefits from the high conductivity and durability of these electrodes:
- Antenna Components: In cellular network antennas and satellite communication systems, these electrodes can be used to create lightweight, highly conductive components.
- Waveguides: The excellent conductivity and corrosion resistance make these materials suitable for use in waveguides for high-frequency communications.
7. Research and Development:
In scientific research, copper-electrodeposited titanium electrodes are valuable tools:
- Electrochemistry Research: These electrodes are used in various electrochemical studies, offering a well-defined surface for experiments.
- Materials Science: The process of creating these electrodes and studying their properties is itself an area of ongoing research in materials science and engineering.
8. Renewable Energy:
In the growing field of renewable energy, these electrodes find applications in:
- Solar Cells: As part of the conductive components in certain types of solar cells, particularly in experimental designs aiming for higher efficiency.
- Wind Turbines: In the electrical systems of wind turbines, where their corrosion resistance and conductivity are beneficial.
9. Automotive Industry:
As the automotive industry moves towards more electric and electronic components, these electrodes find increasing use:
- Electric Vehicle Batteries: In the design of advanced battery systems for electric vehicles, where their lightweight and conductive properties are advantageous.
- Sensor Systems: In various automotive sensor and control systems where conductivity and durability are required.
10. Thermal Management:
The high thermal conductivity of copper combined with the strength of titanium makes these materials useful in thermal management applications:
- Heat Sinks: In high-performance computing and power electronics, where efficient heat dissipation is crucial.
- Thermal Interfaces: As interfaces between different components in systems requiring efficient heat transfer.
The versatility of copper-electrodeposited titanium electrodes continues to drive innovation across these and other industries. As manufacturing techniques improve and new applications are discovered, we can expect to see even more diverse uses for these unique composite materials in the future.
In conclusion, the process of electrodepositing copper using a titanium electrode is a sophisticated technique that combines the beneficial properties of both metals. By carefully controlling the various factors involved in the electrodeposition process, it's possible to create high-quality coatings that meet the specific requirements of a wide range of applications. From electronics and energy storage to aerospace and medical devices, copper-electrodeposited titanium electrodes continue to play a crucial role in advancing technology across numerous industries. As research in this field progresses, we can anticipate even more innovative applications and improved performance of these versatile materials in the future.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Walsh, F. C., & Ponce de León, C. (2014). A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying technology. Transactions of the IMF, 92(2), 83-98.
2. Schlesinger, M., & Paunovic, M. (Eds.). (2011). Modern electroplating (Vol. 55). John Wiley & Sons.
3. Low, C. T. J., Wills, R. G. A., & Walsh, F. C. (2006). Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surface and Coatings Technology, 201(1-2), 371-383.
4. Datta, M., & Landolt, D. (2000). Fundamental aspects and applications of electrochemical microfabrication. Electrochimica Acta, 45(15-16), 2535-2558.
5. Fujimoto, S., Hayashi, S., & Nakao, T. (2001). Improvement of pitting corrosion resistance of titanium by copper electrodeposition. Materials Science and Engineering: A, 308(1-2), 268-273.
6. Eliaz, N., & Gileadi, E. (2008). Physical electrochemistry: fundamentals, techniques, and applications. John Wiley & Sons.
7. Paunovic, M., Schlesinger, M., & Snyder, D. D. (2010). Fundamental considerations. Modern Electroplating, 1-32.