- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Alkaline water electrolysis is a promising technology for producing clean hydrogen fuel, playing a crucial role in the transition towards a sustainable energy future. At the heart of this process lies the electrode-diaphragm assembly, a critical component that determines the efficiency and performance of alkaline electrolyzers. This blog post delves into the intricacies of electrode-diaphragm assemblies, exploring their key components, material considerations, and recent advancements in the field.
What are the key components of an electrode-diaphragm assembly in alkaline water electrolysis?
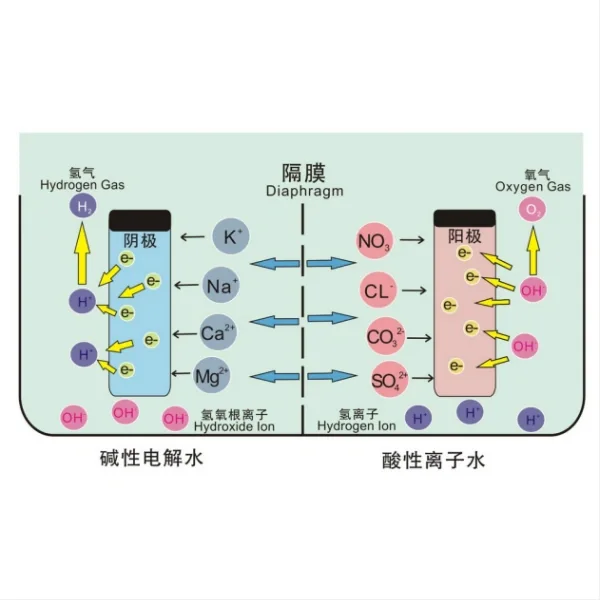
The electrode-diaphragm assembly in alkaline water electrolysis consists of three primary components: the anode, the cathode, and the diaphragm or separator. Each of these elements plays a vital role in the electrolysis process and contributes to the overall efficiency of hydrogen production.
1. Anode: The anode is the positive electrode where the oxygen evolution reaction (OER) occurs. In alkaline electrolysis, water molecules are oxidized at the anode, releasing oxygen gas and hydrogen ions. Common materials used for anodes include nickel, nickel-based alloys, and various metal oxides. These materials are chosen for their high catalytic activity, stability in alkaline environments, and resistance to corrosion.
2. Cathode: The cathode is the negative electrode where the hydrogen evolution reaction (HER) takes place. At the cathode, water molecules are reduced to form hydrogen gas. Nickel and nickel-based alloys are widely used as cathode materials due to their excellent catalytic properties and stability in alkaline conditions. Other materials, such as platinum-group metals and their alloys, are also employed for their high catalytic activity.
3. Diaphragm or Separator: The diaphragm is a crucial component that separates the anode and cathode compartments while allowing the passage of ions. It prevents the mixing of produced gases (oxygen and hydrogen) while facilitating the transport of hydroxide ions. Common materials used for diaphragms include asbestos (although its use is declining due to health concerns), polymeric membranes, and ceramic-based separators.
The design and configuration of these components significantly influence the performance of the electrode-diaphragm assembly. Factors such as electrode surface area, catalyst loading, and diaphragm porosity all play crucial roles in determining the efficiency of the electrolysis process.
How does the choice of materials affect the efficiency of alkaline water electrolysis?
The selection of materials for the electrode-diaphragm assembly is a critical factor in determining the efficiency and durability of alkaline water electrolysis systems. The choice of materials impacts various aspects of the electrolysis process, including catalytic activity, electrical conductivity, and long-term stability.
1. Anode Materials: The anode material must possess high catalytic activity for the oxygen evolution reaction (OER) while maintaining stability in the harsh alkaline environment. Nickel and nickel-based alloys are commonly used due to their excellent performance and cost-effectiveness. However, researchers are continually exploring new materials to enhance OER kinetics. For instance, mixed metal oxides, such as nickel-iron oxides and cobalt-based spinels, have shown promising results in terms of catalytic activity and stability.
2. Cathode Materials: The cathode material should exhibit high catalytic activity for the hydrogen evolution reaction (HER) and resist hydrogen embrittlement. Nickel and its alloys are widely used due to their good catalytic properties and stability. Platinum-group metals, while highly active, are less common in industrial applications due to their high cost. Recent research has focused on developing non-precious metal catalysts, such as nickel-molybdenum alloys and transition metal phosphides, which offer comparable performance at a lower cost.
3. Diaphragm Materials: The choice of diaphragm material affects the ionic conductivity, gas separation efficiency, and overall cell resistance. Traditional asbestos diaphragms are being phased out due to health concerns, leading to the development of alternative materials. Polymeric membranes, such as polysulfone and polyethersulfone, offer good ionic conductivity and mechanical stability. Ceramic-based separators, including zirconium oxide and nickel oxide, provide excellent chemical stability and gas separation properties.
The interplay between these materials significantly influences the overall efficiency of the electrolysis process. For example, the use of high-performance catalysts can reduce the overpotential required for water splitting, thereby increasing energy efficiency. Similarly, the selection of a diaphragm material with low resistance and high ionic conductivity can minimize ohmic losses and improve overall system performance.
Moreover, the long-term stability of these materials is crucial for the economic viability of alkaline electrolyzers. Materials that can withstand the corrosive alkaline environment and maintain their performance over extended periods are highly desirable. This has led to increased research into advanced coatings and surface treatments to enhance the durability of electrode materials.
What recent advancements have been made in electrode-diaphragm assemblies for alkaline electrolyzers?
The field of alkaline water electrolysis has seen significant advancements in recent years, driven by the growing demand for efficient and cost-effective hydrogen production. These innovations have focused on improving the performance, durability, and cost-effectiveness of electrode-diaphragm assemblies.
1. Nanostructured Electrodes: One of the most promising developments in electrode design is the use of nanostructured materials. By increasing the surface area and active sites of electrodes, nanostructured materials can significantly enhance catalytic activity. For instance, nickel nanowires and nanofoams have shown improved performance compared to traditional smooth electrodes. These nanostructured electrodes not only increase the reaction kinetics but also improve gas bubble release, leading to higher overall efficiency.
2. Advanced Catalysts: The development of novel catalysts has been a key focus area for improving electrode performance. Researchers have made significant progress in designing non-precious metal catalysts that can match or even surpass the activity of platinum-group metals. For example, nickel-iron layered double hydroxides have shown exceptional activity for the oxygen evolution reaction, while nickel-molybdenum-based materials have demonstrated high performance for hydrogen evolution. These advancements are crucial for reducing the cost of alkaline electrolyzers while maintaining high efficiency.
3. Composite Diaphragms: To address the limitations of traditional diaphragm materials, researchers have developed composite diaphragms that combine the advantages of different materials. For instance, polymer-ceramic composite membranes offer improved mechanical stability and ionic conductivity compared to single-component diaphragms. These composite materials can be tailored to optimize gas separation, ionic transport, and durability, leading to enhanced overall performance of the electrolysis system.
4. Zero-gap Configuration: A significant advancement in electrode-diaphragm assembly design is the development of zero-gap configurations. In this approach, the electrodes are placed in direct contact with the diaphragm, minimizing the distance between them. This configuration reduces ohmic losses and improves gas separation efficiency, resulting in higher current densities and improved overall system performance. Zero-gap designs have shown promise in increasing the efficiency of alkaline electrolyzers, particularly at high current densities.
5. Additive Manufacturing: The application of 3D printing technologies in electrode fabrication has opened up new possibilities for optimizing electrode structures. Additive manufacturing allows for the creation of complex electrode geometries with precisely controlled porosity and surface area. This enables the design of electrodes with enhanced mass transport properties and improved catalytic activity. 3D-printed electrodes have shown promising results in terms of performance and durability, offering a potential path for cost-effective production of high-performance electrodes.
These advancements in electrode-diaphragm assemblies are driving the development of more efficient, durable, and cost-effective alkaline electrolyzers. As research continues to progress, we can expect further improvements in materials and designs, bringing us closer to the goal of large-scale, economically viable green hydrogen production.

In conclusion, the electrode-diaphragm assembly is a critical component in alkaline water electrolysis, playing a pivotal role in determining the efficiency and performance of hydrogen production systems. By understanding the key components, material considerations, and recent advancements in this field, we can continue to improve the technology and move towards a sustainable hydrogen economy. As research progresses, we can anticipate further innovations that will enhance the efficiency, durability, and cost-effectiveness of alkaline electrolyzers, contributing to the global transition towards clean energy solutions.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Zeng, K., & Zhang, D. (2010). Recent progress in alkaline water electrolysis for hydrogen production and applications. Progress in Energy and Combustion Science, 36(3), 307-326.
2. Schalenbach, M., Tjarks, G., Carmo, M., Lueke, W., Mueller, M., & Stolten, D. (2016). Acidic or alkaline? Towards a new perspective on the efficiency of water electrolysis. Journal of The Electrochemical Society, 163(11), F3197.
3. Pletcher, D., & Li, X. (2011). Prospects for alkaline zero gap water electrolysers for hydrogen production. International Journal of Hydrogen Energy, 36(23), 15089-15104.
4. Xu, W., Scott, K., & Basu, S. (2011). Performance of a high temperature polymer electrolyte membrane water electrolyser. Journal of Power Sources, 196(21), 8918-8924.
5. Marini, S., Salvi, P., Nelli, P., Pesenti, R., Villa, M., Berrettoni, M., ... & Kiros, Y. (2012). Advanced alkaline water electrolysis. Electrochimica Acta, 82, 384-391.
6. Phillips, R., & Dunnill, C. W. (2016). Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Advances, 6(102), 100643-100651.
7. Wang, M., Wang, Z., Gong, X., & Guo, Z. (2014). The intensification technologies to water electrolysis for hydrogen production–A review. Renewable and Sustainable Energy Reviews, 29, 573-588.
8. Ursua, A., Gandia, L. M., & Sanchis, P. (2012). Hydrogen production from water electrolysis: current status and future trends. Proceedings of the IEEE, 100(2), 410-426.
9. Carmo, M., Fritz, D. L., Mergel, J., & Stolten, D. (2013). A comprehensive review on PEM water electrolysis. International Journal of Hydrogen Energy, 38(12), 4901-4934.
10. Rashid, M. M., Al Mesfer, M. K., Naseem, H., & Danish, M. (2015). Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. International Journal of Engineering and Advanced Technology, 4(3), 80-93.
Related Industry Knowledge
- How do Electrode-Diaphragm Assemblies Function in Alkaline Water Electrolysis?
- How are Bipolar Plates Designed for Hydrogen Production Through Water Electrolysis?
- How is Electrode-Diaphragm Assembly Used for Alkaline Water Electrolysis?
- Is Titanium Good for Electrolysis?
- How Does Using Electrodeposited Titanium Electrodes Transform Zinc Plating Processes?
- Why Should You Consider Titanium Electrodes for Copper Plating?