- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
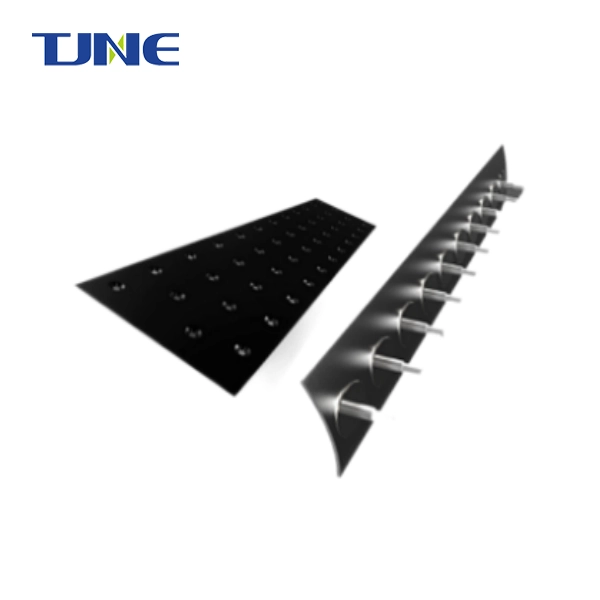
Electrode-diaphragm assembly plays a crucial role in alkaline water electrolysis, a process used to produce hydrogen and oxygen gases from water. This assembly consists of electrodes, typically made of materials like nickel or titanium, and a diaphragm that separates the anodic and cathodic compartments. The design and composition of this assembly significantly impact the efficiency and performance of the electrolysis process. In alkaline water electrolysis, an electric current is passed through the electrodes, causing water molecules to split into hydrogen and oxygen gases. The diaphragm prevents the recombination of these gases while allowing the exchange of ions necessary for the electrochemical reaction.
What are the advantages of using titanium electrodes in water electrolysis?
Titanium electrodes have gained considerable attention in the field of water electrolysis due to their unique properties and advantages. One of the primary benefits of using titanium electrodes is their exceptional corrosion resistance. In the harsh alkaline environment of water electrolysis, many metals would quickly degrade, but titanium forms a stable oxide layer on its surface, protecting it from corrosion and extending its lifespan. This durability makes titanium electrodes a cost-effective choice in the long run, despite their higher initial cost compared to some other electrode materials.
Another advantage of titanium electrodes is their high electrical conductivity. While not as conductive as some metals like copper or silver, titanium offers a good balance between conductivity and corrosion resistance. This property ensures efficient electron transfer during the electrolysis process, contributing to overall system performance. Additionally, titanium has a low density compared to many other metals used in electrodes, making it lighter and easier to handle in large-scale industrial applications.
Titanium electrodes also exhibit excellent stability under varying pH conditions. This characteristic is particularly valuable in alkaline water electrolysis, where the electrolyte solution can have a high pH. The stability of titanium ensures consistent performance across a range of operating conditions, making the electrolysis process more reliable and easier to control.
Furthermore, titanium electrodes can be easily modified or coated to enhance their catalytic properties. For instance, coating titanium electrodes with platinum or other noble metals can significantly improve their electrocatalytic activity, leading to higher efficiency in hydrogen and oxygen production. This versatility in surface modification makes titanium an attractive base material for developing advanced electrode designs.
How does the choice of electrode material affect the efficiency of alkaline water electrolysis?
The choice of electrode material is a critical factor in determining the efficiency of alkaline water electrolysis. Different materials exhibit varying levels of catalytic activity, stability, and electrical conductivity, all of which directly impact the overall performance of the electrolysis system.
Firstly, the catalytic activity of the electrode material plays a crucial role in the efficiency of the water-splitting reaction. Materials with high catalytic activity can lower the activation energy required for the reaction, allowing it to proceed more rapidly and with less electrical input. For example, nickel-based electrodes are commonly used in alkaline electrolysis due to their good catalytic properties and relatively low cost. However, more advanced materials like platinum-group metals or their alloys offer even higher catalytic activity, albeit at a higher cost.
The stability of the electrode material in the alkaline environment is another critical factor. Materials that are prone to corrosion or degradation not only reduce the lifespan of the electrodes but can also contaminate the electrolyte and decrease the system's efficiency over time. This is where materials like titanium shine, as their excellent corrosion resistance ensures long-term stability and consistent performance.
Electrical conductivity of the electrode material also affects efficiency. Materials with higher conductivity reduce electrical resistance within the system, minimizing energy losses and improving overall efficiency. While some highly conductive materials like copper are not suitable due to corrosion issues, others like titanium offer a good balance between conductivity and stability.
The surface area of the electrode is another important consideration. Materials that can be fabricated into high-surface-area structures, such as porous or nanostructured electrodes, provide more active sites for the electrolysis reaction, potentially increasing efficiency. Titanium, for instance, can be easily fabricated into various forms, including mesh or expanded metal structures, to increase its effective surface area.
Moreover, the ability of the electrode material to facilitate the release of gas bubbles affects efficiency. During electrolysis, hydrogen and oxygen bubbles form on the electrode surfaces. If these bubbles are not efficiently released, they can block active sites and increase electrical resistance. Some materials and surface treatments can enhance bubble release, improving the overall efficiency of the process.
What role does the diaphragm play in the electrode-diaphragm assembly for alkaline electrolysis?
The diaphragm in the electrode-diaphragm assembly plays a crucial role in the alkaline electrolysis process, significantly impacting the efficiency, safety, and purity of the produced gases. Understanding its function and characteristics is essential for optimizing the overall performance of the electrolysis system.
Primarily, the diaphragm serves as a physical barrier between the anodic and cathodic compartments of the electrolysis cell. This separation is vital for preventing the mixing of the produced hydrogen and oxygen gases, which could lead to dangerous recombination reactions. By keeping these gases apart, the diaphragm ensures the safety of the electrolysis process and maintains the purity of the produced gases, which is crucial for many applications, particularly in industries requiring high-purity hydrogen.
However, while the diaphragm needs to be an effective barrier for gases, it must also allow for the passage of ions through its structure. In alkaline electrolysis, hydroxide ions (OH-) need to move freely between the electrodes to complete the electrochemical circuit. The diaphragm, therefore, needs to be permeable to these ions while remaining impermeable to gases. This selective permeability is a key characteristic that determines the efficiency of the electrolysis process.
The choice of material for the diaphragm is critical and depends on various factors. Commonly used materials include asbestos (although its use is declining due to health concerns), polymers like polysulfone or polyphenylene sulfide, and ceramic materials. Each of these materials offers different properties in terms of ion conductivity, gas permeability, chemical stability, and mechanical strength.
The thickness of the diaphragm is another important consideration. A thinner diaphragm generally offers lower resistance to ion transport, potentially improving the efficiency of the electrolysis process. However, it must also be thick enough to provide adequate mechanical strength and gas separation. Finding the optimal thickness is a balancing act that depends on the specific requirements of the electrolysis system.
Porosity is another crucial characteristic of the diaphragm. A more porous structure allows for better ion transport but may compromise gas separation if the pores are too large. Advanced diaphragm designs often feature a carefully controlled pore size distribution to optimize both ion transport and gas separation.
The chemical stability of the diaphragm in the highly alkaline environment of the electrolysis cell is also vital. The diaphragm must withstand prolonged exposure to the electrolyte without degrading or losing its selective permeability. This stability ensures consistent performance and longevity of the electrolysis system.
In recent years, there has been significant research into developing advanced diaphragm materials and designs. For instance, composite diaphragms that combine the advantages of different materials are being explored. These might include a porous support structure for mechanical strength coated with a thin, ion-conductive layer for improved performance.
Some researchers are also investigating the use of ion-exchange membranes as an alternative to traditional diaphragms. These membranes can offer higher selectivity in ion transport, potentially leading to improved efficiency and gas purity. However, they often come with higher costs and may have limitations in terms of chemical stability in highly alkaline environments.
The integration of the diaphragm with the electrodes is another area of ongoing development. Some designs feature zero-gap configurations, where the diaphragm is in direct contact with the electrodes. This arrangement can reduce the overall cell resistance and improve efficiency, but it requires careful engineering to ensure proper gas separation and prevent short circuits.
In conclusion, the electrode-diaphragm assembly is a critical component in alkaline water electrolysis, with each element playing a vital role in the efficiency and effectiveness of the process. The choice of electrode material, particularly the use of titanium electrodes, can significantly impact the performance and longevity of the electrolysis system. The diaphragm, while often overlooked, is equally crucial in ensuring the safety, efficiency, and purity of the produced gases. As research in this field continues, we can expect to see further advancements in materials and designs, leading to more efficient and cost-effective alkaline water electrolysis systems. These improvements will be essential in making hydrogen production more economically viable, supporting the growing hydrogen economy and contributing to a more sustainable energy future.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Zeng, K., & Zhang, D. (2010). Recent progress in alkaline water electrolysis for hydrogen production and applications. Progress in Energy and Combustion Science, 36(3), 307-326.
2. Pletcher, D., & Li, X. (2011). Prospects for alkaline zero gap water electrolysers for hydrogen production. International Journal of Hydrogen Energy, 36(23), 15089-15104.
3. Rashid, M. M., Al Mesfer, M. K., Naseem, H., & Danish, M. (2015). Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. International Journal of Engineering and Advanced Technology, 4(3), 80-93.
4. Sapountzi, F. M., Gracia, J. M., Weststrate, C. J., Fredriksson, H. O., & Niemantsverdriet, J. W. (2017). Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Progress in Energy and Combustion Science, 58, 1-35.
5. Marini, S., Salvi, P., Nelli, P., Pesenti, R., Villa, M., Berrettoni, M., ... & Kiros, Y. (2012). Advanced alkaline water electrolysis. Electrochimica Acta, 82, 384-391.
6. Xu, W., & Scott, K. (2010). The effects of ionomer content on PEM water electrolyser membrane electrode assembly performance. International Journal of Hydrogen Energy, 35(21), 12029-12037.
7. Ursua, A., Gandia, L. M., & Sanchis, P. (2012). Hydrogen production from water electrolysis: current status and future trends. Proceedings of the IEEE, 100(2), 410-426.
8. Carmo, M., Fritz, D. L., Mergel, J., & Stolten, D. (2013). A comprehensive review on PEM water electrolysis. International Journal of Hydrogen Energy, 38(12), 4901-4934.
9. Phillips, R., & Dunnill, C. W. (2016). Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Advances, 6(102), 100643-100651.
10. Divisek, J., & Schmitz, H. (1982). A bipolar cell for advanced alkaline water electrolysis. International Journal of Hydrogen Energy, 7(9), 703-710.