- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
How are Bipolar Plates Designed for Hydrogen Production Through Water Electrolysis?
Bipolar plates for PEM electrolyzer are crucial components in water electrolysis systems, playing a pivotal role in the production of hydrogen and oxygen gases. The bipolar plates are electrodes separated by a diaphragm or membrane, which allows for the efficient splitting of water molecules into their constituent elements. The design of the bipolar plate is critical for optimizing the electrolysis process, enhancing energy efficiency, and ensuring long-term durability of the system. In this blog post, we'll explore the key aspects of bipolar plate design for water electrolysis, including material selection, efficiency considerations, and scaling challenges.
What materials are used in hydrogen production through water electrolysis?
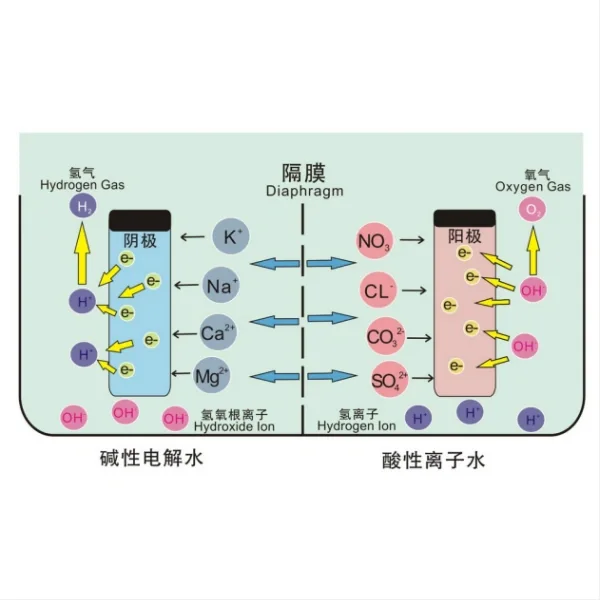
Bipolar plates are flat thick metal separators with etched flow channels, used to connect multiple electrolyzer cells in series to match the power supply voltage. They separate adjacent cells and provide electronic connectivity. They require low resistance and high mechanical and chemical stability, fluid distribution, and high thermal conductivity because they also help facilitate heat transfer. Titanium is generally considered the most advanced material due to its excellent strength, low resistivity, high thermal conductivity, and low hydrogen permeability. However, titanium is prone to corrosion, leading to the accumulation of surface oxides that increase contact resistance and reduce thermal conductivity. To prevent this, a thin platinum coating can be applied to reduce surface resistance.

Diaphragm Materials
The diaphragm serves to separate the evolved gases while allowing ion transport. Key materials include:
1. Asbestos: Traditionally used due to its excellent stability, but being phased out due to health concerns.
2. Ceramic materials: Various ceramic compositions, such as those based on nickel oxide or zirconium oxide, provide good stability and separation properties.
3. Polymer-based membranes: Advanced polymer membranes, including those based on polysulfone or polyethersulfone, are being developed to offer improved performance and durability.
The selection of these materials must consider factors such as electrical conductivity, catalytic activity, corrosion resistance, and cost-effectiveness. Ongoing research focuses on developing novel materials and coatings to enhance the performance and longevity of electrode-diaphragm assemblies in water electrolysis systems.
How does the design of bipolar plates affect efficiency in water electrolysis?
The design of bipolar plates plays a crucial role in determining the overall efficiency of water electrolysis systems. Several key design aspects significantly impact the performance:
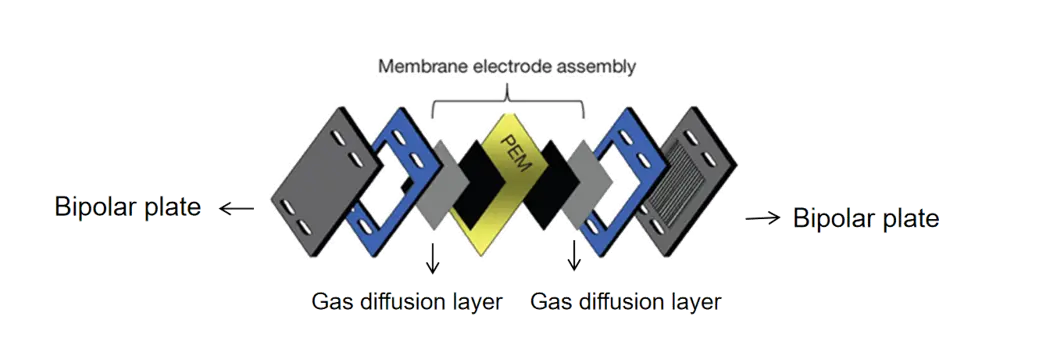
Electrode Surface Area and Morphology
1. Increased surface area: Designing electrodes with high surface area, such as through the use of porous structures or nanostructured materials, enhances the number of active sites for electrocatalysis. This leads to improved reaction kinetics and higher current densities.
2. Surface roughness: Controlled surface roughness can increase the effective surface area and create more catalytically active sites. However, excessive roughness may lead to gas bubble entrapment, which can hinder efficiency.
3. Electrode thickness: Optimizing electrode thickness is crucial for balancing electrical conductivity and mass transport. Thicker electrodes may offer better conductivity but can impede the diffusion of reactants and products.
Inter-electrode Gap
The distance between the anode and cathode, known as the inter-electrode gap, significantly affects the ohmic resistance of the electrolyte:
1. Narrow gap: A smaller gap reduces the ohmic drop in the electrolyte, leading to lower cell voltage and improved energy efficiency. However, it may increase the risk of gas crossover.
2. Gap optimization: The optimal gap must balance reduced ohmic resistance with adequate space for gas evolution and separation.
Gas Separation and Management
Efficient gas separation is crucial for maintaining high system efficiency and product purity:
1. Diaphragm porosity: The porosity of the diaphragm must be carefully controlled to allow ion transport while preventing gas crossover.
2. Gas release channels: Designing electrodes with channels or structures that facilitate easy gas release can prevent bubble accumulation and reduce ohmic resistance.
3. Flow field design: Implementing flow fields in the electrode structure can enhance mass transport and gas removal, improving overall efficiency.
Current Distribution
Uniform current distribution across the electrode surface is essential for optimal performance:
1. Electrode shape and size: The geometry of the electrodes should be designed to promote even current distribution and avoid "hot spots" that can lead to local performance degradation.
2. Current collectors: Proper design of current collectors ensures efficient electron transfer and uniform current distribution across the entire electrode surface.
Temperature Management
Temperature plays a significant role in electrolysis efficiency:
1. Heat exchange systems: Incorporating efficient heat exchange mechanisms can help maintain optimal operating temperatures, as higher temperatures generally improve kinetics and reduce ohmic losses.
2. Thermal gradients: Designing the assembly to minimize thermal gradients can prevent local performance variations and potential material stress.
Pressure Considerations
Operating pressure can affect system efficiency:
1. Pressurized designs: Higher pressure operation can reduce the energy required for subsequent gas compression but may increase the complexity of sealing and materials requirements.
2. Differential pressure management: Designing the assembly to handle potential pressure differences between the anode and cathode compartments is crucial for long-term stability.
Scalability and Modularity
Efficient large-scale systems often rely on modular designs:
1. Stack configurations: Designing bipolar plates that can be easily stacked and connected in series or parallel allows for flexible scaling of electrolysis capacity.
2. Uniform flow distribution: Ensuring uniform electrolyte and gas flow distribution across multiple cells in a stack is crucial for maintaining efficiency at scale.
By carefully considering these design aspects, engineers can significantly enhance the efficiency of water electrolysis systems. Ongoing research continues to push the boundaries of bipolar plates design, seeking to improve performance, reduce costs, and increase the viability of large-scale hydrogen production through water electrolysis.
What are the challenges in scaling up bipolar plates for industrial water electrolysis?
As the demand for green hydrogen production grows, scaling up water electrolysis systems for industrial applications presents several significant challenges. These challenges primarily revolve around maintaining efficiency, ensuring long-term durability, and managing costs at larger scales. Here are the key issues faced when scaling up bipolar plates for PEM electrolyzer:
1. Maintaining Performance at Scale
One of the primary challenges is ensuring that the high performance achieved in smaller-scale systems translates to larger industrial setups:
- Uniform current distribution: As electrode size increases, maintaining uniform current distribution becomes more difficult. Non-uniform current can lead to hotspots, reduced efficiency, and accelerated degradation of components.
- Mass transport limitations: Larger systems may face challenges in efficiently supplying reactants to and removing products from the electrode surfaces, potentially limiting overall system performance.
- Heat management: Larger systems generate more heat, requiring more sophisticated cooling mechanisms to maintain optimal operating temperatures across the entire assembly.
2. Material Constraints and Costs
Scaling up production often reveals limitations in material availability and cost-effectiveness:
- Rare and expensive materials: Some high-performance electrode materials, such as platinum group metals, become prohibitively expensive at industrial scales.
- Supply chain issues: Ensuring a stable supply of specialized materials for large-scale production can be challenging, especially for novel or advanced materials.
- Balancing performance and cost: Finding the right balance between using high-performance materials and keeping costs manageable is crucial for commercial viability.

3. Manufacturing and Quality Control
Producing large-scale bipolar plates for PEM electrolyzer presents its own set of challenges:
- Consistent quality: Maintaining consistent quality across large electrode surfaces and between multiple units is challenging but crucial for overall system performance.
- Advanced manufacturing techniques: Scaling up may require the development of new manufacturing processes capable of producing large, complex electrode structures with high precision.
- Quality control: Implementing effective quality control measures for large-scale production is essential but can be more complex and time-consuming.
4. Durability and Lifetime
Ensuring long-term durability becomes increasingly critical at industrial scales:
- Accelerated degradation: Factors that might cause minor degradation in small-scale systems can lead to significant performance losses and increased maintenance needs in large-scale operations.
- Stress and fatigue: Larger components may be more susceptible to mechanical stress and fatigue, especially under the dynamic conditions of industrial operation.
- Corrosion resistance: Maintaining corrosion resistance over large surface areas and extended operating periods is crucial for long-term performance.
5. System Integration and Balance of Plant
Scaling up requires careful consideration of how the bipolar plates integrate with other system components:
- Electrolyte management: Large-scale systems require more complex electrolyte circulation and management systems to maintain optimal concentrations and remove impurities.
- Gas handling: Efficient collection, purification, and storage of produced gases become more challenging at larger scales.
- Power supply: Designing and integrating power supply systems capable of delivering high currents efficiently across large electrode areas is crucial.
6. Safety and Environmental Considerations
Larger systems inherently pose greater safety and environmental risks:
- Gas management: Handling large volumes of hydrogen and oxygen safely requires robust safety systems and protocols.
- Environmental impact: Minimizing the environmental footprint of large-scale production, including water usage and potential chemical releases, becomes more critical.
- Regulatory compliance: Meeting safety and environmental regulations for large-scale industrial operations can be more complex and stringent.
7. Flexibility and Responsiveness
Industrial-scale systems often need to operate under varying conditions:
- Load following: Designing systems that can efficiently operate under variable loads to match fluctuating renewable energy inputs or hydrogen demand is challenging.
- Start-up and shutdown: Optimizing large systems for frequent start-ups and shutdowns without compromising performance or longevity is crucial for integration with intermittent renewable energy sources.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, electrochemistry, engineering, and manufacturing. Ongoing research and development efforts are focused on innovative electrode and diaphragm materials, advanced manufacturing techniques, and improved system designs to overcome these scaling challenges. As these solutions are developed and implemented, they will play a crucial role in making large-scale alkaline water electrolysis a viable technology for industrial-scale green hydrogen production.
In conclusion, the design of bipolar plates for electrolysis is a complex and multifaceted challenge that requires careful consideration of materials, efficiency, and scalability. As research progresses and technology advances, we can expect to see significant improvements in the performance, durability, and cost-effectiveness of these systems, paving the way for more widespread adoption of green hydrogen production.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References
1. Zeng, K., & Zhang, D. (2010). Recent progress in alkaline water electrolysis for hydrogen production and applications. Progress in Energy and Combustion Science, 36(3), 307-326.
2. Pletcher, D., & Li, X. (2011). Prospects for alkaline zero gap water electrolysers for hydrogen production. International Journal of Hydrogen Energy, 36(23), 15089-15104.
3. Schalenbach, M., Tjarks, G., Carmo, M., Lueke, W., Mueller, M., & Stolten, D. (2016). Acidic or alkaline? Towards a new perspective on the efficiency of water electrolysis. Journal of The Electrochemical Society, 163(11), F3197-F3208.
4. Phillips, R., & Dunnill, C. W. (2016). Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Advances, 6(102), 100643-100651.
5. Manabe, A., Kashiwase, M., Hashimoto, T., Hayashida, T., Kato, A., Hirao, K., ... & Shimomura, I. (2013). Basic study of alkaline water electrolysis. Electrochimica Acta, 100, 249-256.
6. Ursua, A., Gandia, L. M., & Sanchis, P. (2012). Hydrogen production from water electrolysis: current status and future trends. Proceedings of the IEEE, 100(2), 410-426.
7. Mazloomi, K., & Gomes, C. (2012). Hydrogen as an energy carrier: Prospects and challenges. Renewable and Sustainable Energy Reviews, 16(5), 3024-3033.
8. Carmo, M., Fritz, D. L., Mergel, J., & Stolten, D. (2013). A comprehensive review on PEM water electrolysis. International Journal of Hydrogen Energy, 38(12), 4901-4934.
9. Diéguez, P. M., Ursúa, A., Sanchis, P., Sopena, C., Guelbenzu, E., & Gandía, L. M. (2008). Thermal performance of a commercial alkaline water electrolyzer: Experimental study and mathematical modeling. International Journal of Hydrogen Energy, 33(24), 7338-7354.
10. Rashid, M. M., Al Mesfer, M. K., Naseem, H., & Danish, M. (2015). Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. International Journal of Engineering and Advanced Technology, 4(3), 80-93.
Related Industry Knowledge
- What is the Consumption Rate of MMO Anode Plates?
- What is Platinum Plated Titanium?
- How Do Titanium Anodes Contribute to the Innovation in Cobalt Electrowinning Technology?
- Are There Environmental Benefits to Using Titanium Anodes for Copper Electrowinning?
- Electrochemical Essentials: The Comprehensive Guide to Anode Plates
- Which Industries Utilize MMO Anode Plates for Corrosion Protection and Cathodic Protection?
- What Is an MMO Anode Plate and How Does It Function in Electrochemical Processes?