- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
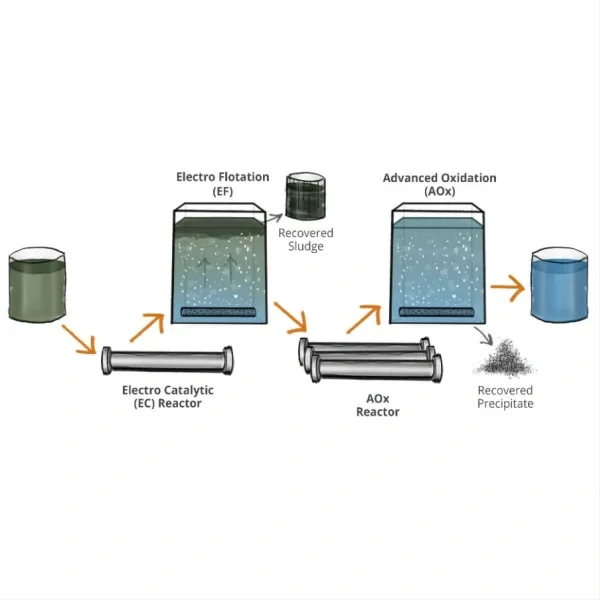
Titanium electrodes have emerged as a game-changing technology in the field of seawater electrolysis, offering significant improvements in efficiency and durability. As the global demand for clean hydrogen fuel continues to rise, researchers and engineers are increasingly turning to seawater electrolysis as a sustainable method of production. In this context, titanium electrodes play a crucial role in enhancing the overall process efficiency, making it more economically viable and environmentally friendly.
What are the advantages of using titanium electrodes in seawater electrolysis?
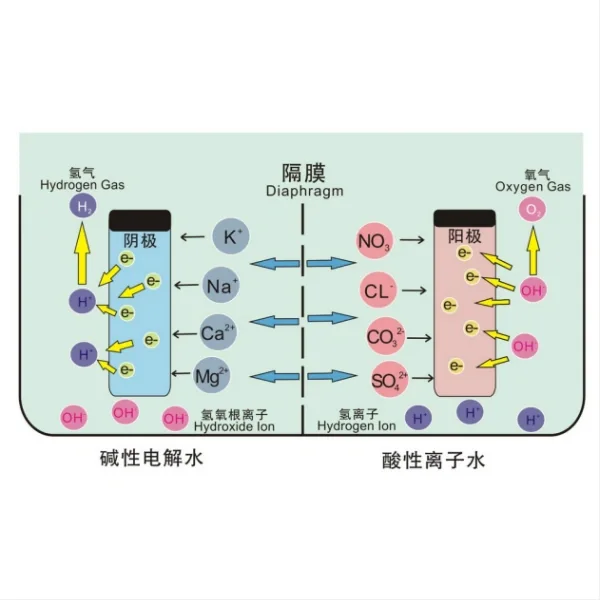
Titanium electrodes offer several key advantages that make them particularly well-suited for seawater electrolysis applications. First and foremost is their exceptional corrosion resistance. Seawater is a highly corrosive environment due to its high salt content and the presence of various dissolved minerals. Traditional electrode materials, such as stainless steel or carbon, can quickly degrade in these conditions, leading to reduced efficiency and frequent replacements. Titanium, on the other hand, forms a stable passive oxide layer when exposed to oxygen, providing excellent protection against corrosion even in harsh marine environments.
Another significant advantage of titanium electrodes is their high electrical conductivity. While pure titanium is not as conductive as some other metals, it can be alloyed or coated with more conductive materials to enhance its performance. For example, titanium electrodes are often coated with platinum group metals or mixed metal oxides, creating a surface that combines the corrosion resistance of titanium with the excellent catalytic properties of these materials. This results in electrodes that can efficiently conduct electricity while maintaining their structural integrity over long periods of use.

Titanium electrodes also boast impressive mechanical strength and durability. In seawater electrolysis systems, electrodes are subjected to various stresses, including mechanical vibrations, temperature fluctuations, and pressure changes. The high strength-to-weight ratio of titanium ensures that the electrodes can withstand these challenging conditions without deforming or breaking. This durability translates to longer operational lifetimes and reduced maintenance requirements, which are crucial factors in large-scale industrial applications.
Furthermore, titanium electrodes have a low hydrogen overpotential, which is a critical factor in electrolysis efficiency. The overpotential represents the additional voltage required above the theoretical minimum to drive the electrolysis reaction. A lower overpotential means less energy is wasted as heat, resulting in higher overall energy efficiency. By minimizing this energy loss, titanium electrodes contribute significantly to the economic viability of seawater electrolysis.
How does the surface treatment of titanium electrodes affect their performance in seawater electrolysis?
1. Enhanced Electrochemical Reactions
The surface treatment of titanium electrodes involves applying a thin coating of mixed metal oxides. This coating is crucial because it significantly enhances the electrochemical reactions that occur during electrolysis.
- Mixed Metal Oxide Coating: The coating typically consists of a proprietary blend of oxides such as ruthenium, iridium, tin, and other electrocatalysts. These materials are chosen for their excellent electrocatalytic properties.
- Lowered Overpotential: The mixed metal oxide coating lowers the overpotential required for the electrolysis process. Overpotential is the extra voltage needed above the thermodynamically calculated value to drive an electrochemical reaction. By reducing this, the electrode can operate more efficiently at lower voltages.
- Improved Activation Kinetics: The coating improves the activation kinetics of the electrolytic reactions. This means that the reactions occur more rapidly and efficiently, leading to quicker generation of chlorine and other reactive species.
2. Increased Chlorine Generation Efficiency
Chlorine generation is a key outcome of the electrolysis process in ballast water treatment. The surface treatment directly influences this efficiency.
- Efficient Chlorine Production: The mixed metal oxide coating is optimized for chlorine generation. It ensures that the electrolysis of seawater produces a high yield of chlorine, which is essential for effective disinfection.
- Low Voltage Operation: Due to the improved electrocatalytic properties, the electrode can generate chlorine efficiently even at low voltages. This not only saves energy but also reduces the risk of unwanted side reactions that could occur at higher voltages.
3. Corrosion Resistance
Titanium is already known for its exceptional corrosion resistance, but the surface treatment further enhances this property.
- Durable Performance: The mixed metal oxide coating provides an additional layer of protection against corrosion. This ensures that the electrode maintains its performance over extended periods, even in the highly corrosive environment of seawater.
- Long-Lasting Efficiency: The durability of the coating means that the electrode can sustain high disinfection efficiency without degradation, ensuring reliable operation in demanding conditions.
4. High Current Density Support
The surface treatment allows the titanium electrode to support high current densities.
- Enhanced Electrolysis Efficiency: High current densities mean that more electrochemical reactions can occur simultaneously, leading to a more efficient and quicker treatment process.
- Compact and Effective Design: The ability to operate at high current densities also allows for a more compact electrode design. This is particularly beneficial in marine applications where space is often limited.
What are the challenges and future prospects of using titanium electrodes in large-scale seawater electrolysis?
Challenges:
Cost of Materials: The text mentions the use of "grade 1 titanium" and a "proprietary blend" of oxides including ruthenium and iridium. These materials are expensive. Scaling up to large-scale seawater electrolysis would significantly increase material costs, potentially making the process economically unviable without cost reductions. The text doesn't address cost, but it's a major hurdle for widespread adoption.
Coating Durability & Degradation: While titanium itself is corrosion-resistant, the mixed metal oxide coating is crucial for efficient chlorine generation. The text doesn't detail the long-term stability of this coating. In large-scale, continuous operation, these coatings can degrade over time due to corrosion, dissolution, or mechanical wear. This degradation reduces efficiency and necessitates replacement, adding to operational costs. The text highlights durability, but doesn't quantify it or address long-term performance in harsh, large-scale conditions.
Fouling & Biofilm Formation: Seawater is a complex environment. Large-scale systems are prone to biofouling (the accumulation of microorganisms on the electrode surface) and the formation of biofilms. This reduces the effective surface area for electrolysis, lowers efficiency, and increases energy consumption. The text focuses on killing microorganisms in the water, but doesn't address preventing their attachment to the electrode.
Scale-Up Issues: Optimizing electrode design for a small ballast water system is different than for a massive industrial-scale electrolyzer. Maintaining uniform current distribution, managing heat generation, and ensuring consistent performance across a large electrode surface area present significant engineering challenges.
Energy Consumption: While the text mentions high current densities and efficiency, it doesn't provide specific energy consumption figures. Large-scale electrolysis requires substantial energy input. Improving energy efficiency is critical for economic and environmental sustainability.
Future Prospects:
Advanced Coating Development: Research into more durable, cost-effective, and highly active mixed metal oxide coatings is crucial. This includes exploring alternative materials to ruthenium and iridium (which are rare and expensive) and developing coatings with self-healing properties.
Nanomaterials & Surface Engineering: Utilizing nanomaterials and advanced surface engineering techniques to enhance the catalytic activity and stability of the electrode coatings. This could involve creating nanostructured surfaces to maximize surface area and improve electron transfer.
Electrode Design Optimization: Developing innovative electrode geometries and configurations to improve current distribution, reduce energy consumption, and minimize fouling. This could include 3D-printed electrodes or novel flow-through designs.
Hybrid Systems: Combining titanium electrode electrolysis with other disinfection technologies (e.g., UV irradiation, filtration) to create hybrid systems that offer synergistic benefits and reduce reliance on high chlorine concentrations.
Integration with Renewable Energy: Coupling large-scale seawater electrolysis with renewable energy sources (solar, wind) to create a sustainable and environmentally friendly process. This would address the energy consumption challenge.
Electrocatalyst Recycling: Developing methods to recover and recycle the valuable metals (ruthenium, iridium) from spent electrode coatings, reducing costs and promoting circular economy principles.
Monitoring & Control Systems: Implementing advanced monitoring and control systems to optimize electrode performance, detect fouling, and adjust operating parameters in real-time.
In conclusion, while challenges remain in scaling up titanium electrode technology for large-scale seawater electrolysis, the ongoing research and development in this field, coupled with the increasing demand for sustainable hydrogen production, paint a promising picture for the future. As we continue to innovate and refine these technologies, titanium electrodes are poised to play a crucial role in unlocking the vast potential of seawater as a renewable hydrogen source, contributing significantly to the global transition towards a cleaner energy future.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References
1. Dionigi, F., Reier, T., Pawolek, Z., Gliech, M., & Strasser, P. (2016). Design Criteria, Operating Conditions, and Nickel-Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis. ChemSusChem, 9(9), 962-972.
2. Fujimura, K., Matsui, T., Izumiya, K., Ebara, N., Nagai, E., Sakai, T., ... & Inoue, K. (2019). Oxygen evolution on manganese-based oxides in seawater electrolysis. Catalysts, 9(6), 490.
3. Gong, M., & Dai, H. (2015). A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Research, 8(1), 23-39.
4. Kuang, Y., Kenney, M. J., Meng, Y., Hung, W. H., Liu, Y., Huang, J. E., ... & Dai, H. (2019). Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proceedings of the National Academy of Sciences, 116(14), 6624-6629.
5. Lu, X., Pan, J., Lovell, E., Tan, T. H., Ng, Y. H., & Amal, R. (2018). A sea-change: manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy & Environmental Science, 11(7), 1898-1910.
6. Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
7. Pourbaix, M. (1974). Atlas of electrochemical equilibria in aqueous solutions. National Association of Corrosion Engineers.
8. Schalenbach, M., Tjarks, G., Carmo, M., Lueke, W., Mueller, M., & Stolten, D. (2016). Acidic or alkaline? Towards a new perspective on the efficiency of water electrolysis. Journal of The Electrochemical Society, 163(11), F3197.
9. Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
10. Yu, L., Zhu, Q., Song, S., McElhenny, B., Wang, D., Wu, C., ... & Chen, S. (2019). Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nature Communications, 10(1), 1-10.
Related Industry Knowledge
- Why are titanium electrodes used specifically for ballast water management?
- Why is Titanium Used as Anode in Electrolysis of Brine?
- Why is Titanium Mesh Used?
- Can Titanium Electrodes Be Used with Saltwater Chlorinators?
- Can Titanium Electrodes be Used for Large-Scale Water Treatment?
- How Do Titanium Anodes Enhance Zinc Electrowinning Processes?
- Why MMO Titanium Probe Anodes Are Essential for Advanced Corrosion Protection?