- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
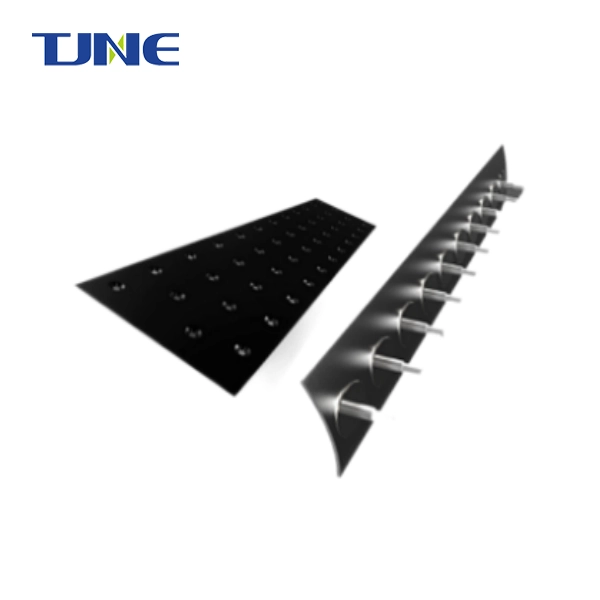
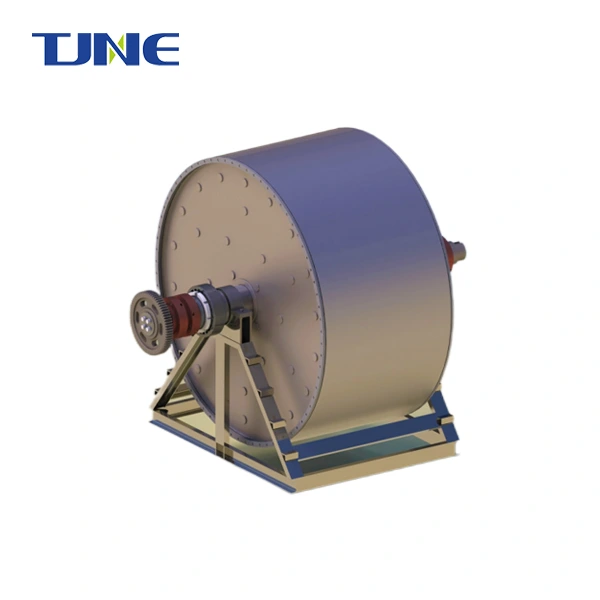
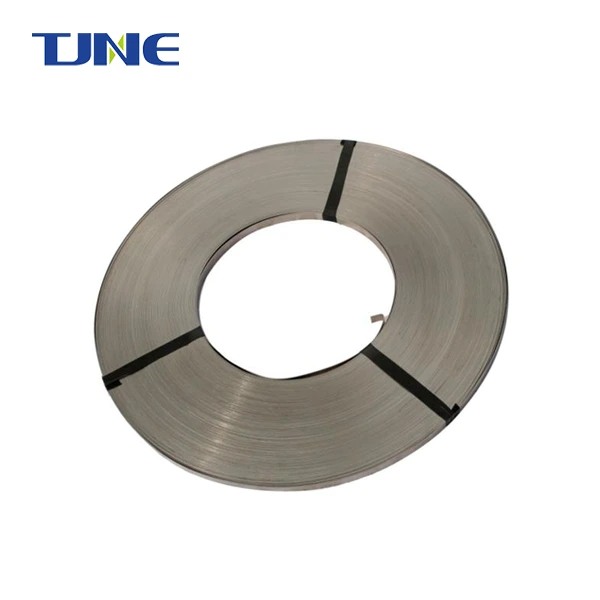
Titanium electrodes play a crucial role in ballast water management systems, particularly in electrochlorination processes. These electrodes are specifically chosen for their unique properties that make them ideal for the harsh marine environment and the demanding task of treating ballast water. Titanium's excellent corrosion resistance, durability, and electrochemical properties make it an optimal material for electrodes in ballast water treatment systems. This blog post will explore the reasons behind the use of titanium electrodes in ballast water management and address some common questions related to this technology.
How do titanium electrodes work in ballast water treatment systems?
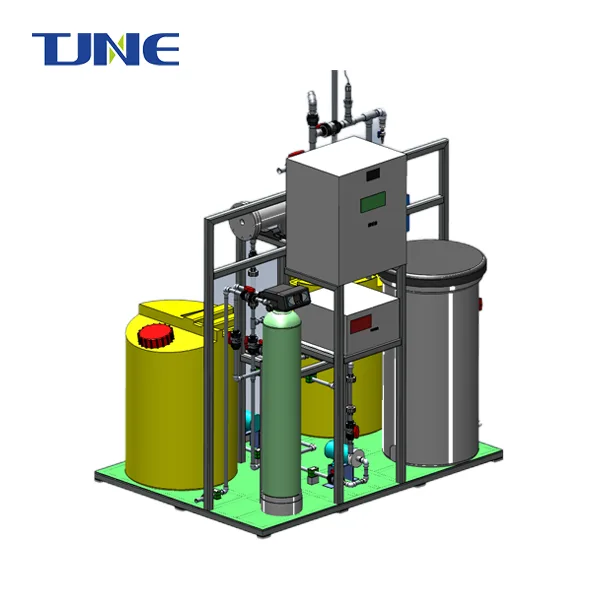
Titanium electrodes are at the heart of electrochlorination systems used in ballast water treatment. These systems utilize the process of electrolysis to generate disinfectants directly from seawater, eliminating the need for storing hazardous chemicals onboard ships. Here's how titanium electrodes work in this context:
Electrolysis process: When an electric current is passed through seawater between titanium electrodes, it causes a chemical reaction. This reaction splits water molecules and salt (sodium chloride) present in seawater into their constituent elements.
Chlorine generation: At the anode (positively charged electrode), chloride ions from salt are oxidized to form chlorine gas. This chlorine immediately reacts with water to form hypochlorite, a powerful disinfectant.
Hydrogen production: At the cathode (negatively charged electrode), water molecules are reduced to form hydrogen gas and hydroxide ions.
Disinfection: The generated hypochlorite acts as a potent biocide, effectively killing or inactivating various marine organisms present in the ballast water, including bacteria, viruses, and larger organisms like plankton.
Titanium's role: Titanium electrodes, particularly those coated with noble metals like platinum or iridium, serve as dimensionally stable anodes (DSA). These electrodes can withstand the corrosive environment and maintain their shape and effectiveness over extended periods.
Efficiency and longevity: The use of titanium electrodes ensures high efficiency in chlorine generation and extends the lifespan of the treatment system. Titanium's resistance to corrosion means that these electrodes can operate effectively for years, even in the harsh saline environment of seawater.
By utilizing titanium electrodes, ballast water treatment systems can provide a reliable, efficient, and environmentally friendly method of disinfecting ballast water, helping ships comply with international regulations while minimizing the risk of introducing invasive species to new ecosystems.
What are the advantages of using titanium electrodes over other materials?
Titanium electrodes offer several significant advantages over other materials when used in ballast water management systems. These benefits contribute to their widespread adoption in the maritime industry:
Corrosion resistance: One of the primary advantages of titanium electrodes is their exceptional resistance to corrosion. Seawater is highly corrosive due to its high salt content and the presence of various minerals. Titanium forms a stable, protective oxide layer on its surface when exposed to oxygen, making it highly resistant to corrosion in marine environments. This property ensures that titanium electrodes maintain their structural integrity and performance over long periods, even under continuous exposure to seawater.
Durability and longevity: The corrosion resistance of titanium translates directly into enhanced durability and longevity of the electrodes. While other materials may degrade quickly in seawater, titanium electrodes can last for years without significant deterioration. This longevity reduces the need for frequent replacements, lowering maintenance costs and minimizing system downtime.
High electrical conductivity: Titanium offers good electrical conductivity, especially when coated with noble metals like platinum or iridium. This property is crucial for efficient electrolysis, ensuring that the electrical energy is effectively used to generate chlorine for water treatment.
Chemical stability: Titanium electrodes remain stable under the varying chemical conditions present in ballast water treatment systems. They do not react unfavorably with the chlorine or other chemical species produced during the electrolysis process, maintaining their effectiveness throughout the treatment cycle.
Lightweight yet strong: Compared to some other electrode materials, titanium offers an excellent strength-to-weight ratio. This property is particularly beneficial in maritime applications where weight considerations are important. The lightweight nature of titanium doesn't compromise its strength, allowing for robust electrode designs that can withstand the mechanical stresses in ballast water systems.
Biocompatibility: Titanium is known for its biocompatibility, meaning it does not have adverse effects on living organisms. This property is important in ballast water treatment, as it ensures that the electrode material itself does not introduce harmful substances into the treated water.
Versatility in design: Titanium can be easily fabricated into various shapes and sizes, allowing for flexible electrode designs to suit different ballast water treatment system configurations. This versatility enables manufacturers to optimize electrode geometry for maximum efficiency and effectiveness.
Temperature resistance: Titanium electrodes can operate effectively across a wide range of temperatures. This is crucial for ballast water treatment systems that may need to function in various climatic conditions, from tropical waters to colder regions.
Low maintenance requirements: Due to their durability and resistance to fouling, titanium electrodes typically require less maintenance compared to electrodes made from other materials. This reduces operational costs and minimizes the need for system shutdowns for electrode maintenance or replacement.
Environmentally friendly: The long lifespan and recyclability of titanium make it an environmentally friendly choice for electrode materials. The reduced need for replacement and the potential for recycling at the end of their life cycle contribute to the overall sustainability of ballast water treatment systems using titanium electrodes.
These advantages collectively make titanium electrodes the preferred choice for ballast water management systems. Their ability to withstand the harsh conditions of seawater while maintaining high performance and efficiency over extended periods justifies their use despite the higher initial cost compared to some alternative materials.
How do titanium electrodes contribute to the efficiency of ballast water treatment?
Titanium electrodes play a crucial role in enhancing the efficiency of ballast water treatment systems, particularly in electrochlorination processes. Their contribution to system efficiency can be observed in several key areas:
High chlorine generation efficiency: Titanium electrodes, especially when coated with noble metals, exhibit high efficiency in chlorine generation. The stable surface of these electrodes provides an ideal platform for the oxidation of chloride ions to chlorine gas, which then forms hypochlorite in water. This high efficiency ensures that a maximum amount of disinfectant is produced for a given input of electrical energy, optimizing the treatment process.
Consistent performance over time: Unlike some other electrode materials that may degrade or lose efficiency over time due to corrosion or scaling, titanium electrodes maintain consistent performance throughout their lifespan. This consistency ensures that the ballast water treatment system operates at peak efficiency for extended periods, reducing the need for frequent adjustments or maintenance.
Low energy consumption: The high electrical conductivity of titanium electrodes, combined with their resistance to passivation (the formation of insulating layers on the electrode surface), contributes to lower energy consumption in the electrolysis process. This energy efficiency is crucial for shipboard operations where power management is a significant concern.
Rapid disinfection: The efficient production of chlorine-based disinfectants by titanium electrodes enables rapid treatment of ballast water. This quick action is essential in maritime operations where time is often a critical factor during ballasting and de-ballasting processes.
Minimal by-product formation: Titanium electrodes, when properly designed and operated, can minimize the formation of unwanted by-products during the electrolysis process. This efficiency in targeting the desired reactions (chlorine generation) while minimizing side reactions contributes to the overall effectiveness and safety of the treatment process.
Adaptability to water conditions: Titanium electrodes perform efficiently across a range of water conditions, including variations in salinity, temperature, and pH. This adaptability ensures that the ballast water treatment system remains effective in different marine environments, maintaining compliance with regulations regardless of the ship's operational area.
Enhanced flow dynamics: The design flexibility of titanium electrodes allows for the creation of optimized electrode geometries. These designs can improve the flow dynamics within the treatment cell, ensuring uniform distribution of the generated disinfectant and maximizing contact with microorganisms in the ballast water.
Reduced maintenance downtime: The durability and corrosion resistance of titanium electrodes significantly reduce the frequency of maintenance and replacement. This minimizes system downtime, allowing for more continuous and efficient operation of the ballast water treatment system.
Scalability: Titanium electrodes can be effectively scaled to suit different system sizes and treatment capacities. This scalability ensures that the efficiency of the treatment process is maintained whether dealing with small or large volumes of ballast water.
Long-term cost efficiency: While the initial cost of titanium electrodes may be higher than some alternatives, their long lifespan and consistent performance contribute to long-term cost efficiency. The reduced need for replacement and maintenance, combined with consistent operational efficiency, often results in lower total cost of ownership over the life of the system.
By contributing to these aspects of system performance, titanium electrodes significantly enhance the overall efficiency of ballast water treatment processes. Their use enables ship operators to effectively comply with stringent environmental regulations while optimizing operational costs and minimizing environmental impact.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Tsolaki, E., & Diamadopoulos, E. (2010). Technologies for ballast water treatment: a review. Journal of Chemical Technology & Biotechnology, 85(1), 19-32.
2. Werschkun, B., et al. (2014). Emerging risks from ballast water treatment: The run-up to the International Ballast Water Management Convention. Chemosphere, 112, 256-266.
3. Mouchtouri, V. A., et al. (2014). Efficacy of various disinfectants used in ship's ballast water treatment systems. Environmental Technology, 35(17-20), 2140-2151.
4. Batista, W. R., et al. (2017). Which ballast water management system will you put aboard? Remnant anxieties: A mini-review. Environments, 4(3), 54.
5. Delacroix, S., et al. (2013). Disinfection by-products and ecotoxicity of ballast water after oxidative treatment – Results and experiences from seven years of full-scale testing of ballast water management systems. Marine Pollution Bulletin, 73(1), 24-36.
6. Balaji, R., et al. (2011). Characteristics of biosecure ballast water treatment systems. Marine Technology Society Journal, 45(4), 96-103.
7. Čampara, L., et al. (2019). Overview and comparison of the IMO and the US maritime administration ballast water management regulations. Journal of Marine Science and Engineering, 7(9), 283.
8. Martínez, L. F., et al. (2018). Disinfection technologies for ballast water: Laboratory-scale study. Journal of Marine Science and Engineering, 6(4), 147.
9. Bradie, J., et al. (2018). A microfluidic device for high-throughput viability assessment of microalgae. Algal Research, 31, 442-451.
10. Pereira, N. N., & Brinati, H. L. (2012). Onshore ballast water treatment: A viable option for major ports. Marine Pollution Bulletin, 64(11), 2296-2304.