- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
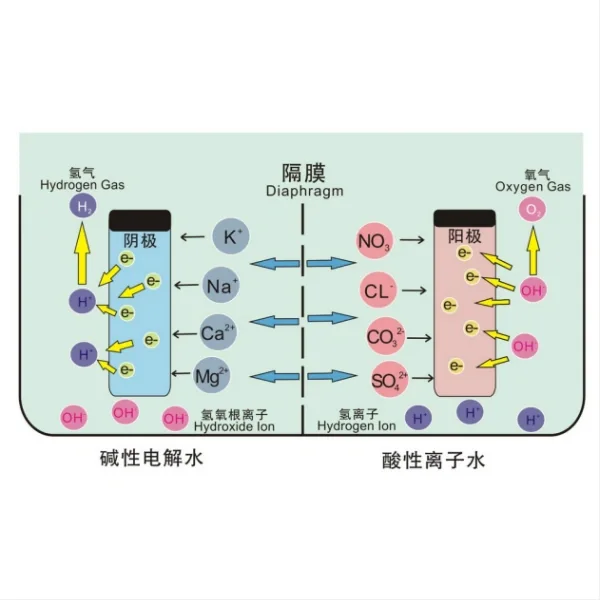
Ruthenium Iridium Coated Titanium Anodes, often referred to as Mixed Metal Oxide (MMO) anodes, are advanced electrochemical components that play a crucial role in various industrial processes. These anodes consist of a titanium substrate coated with a mixture of ruthenium and iridium oxides. The unique combination of these materials creates a highly efficient and durable electrode that excels in electrochemical applications.
The working principle of Ruthenium Iridium Coated Titanium Anodes is based on their ability to facilitate electron transfer reactions at the electrode-electrolyte interface. The ruthenium and iridium oxide coating provides a catalytic surface that enhances the rate of electrochemical reactions, particularly the oxidation of water to produce oxygen. This process is fundamental in many industrial applications, including chlorine production, water treatment.
The titanium substrate serves as an excellent base material due to its corrosion resistance and mechanical strength. The ruthenium and iridium oxides form a stable, conductive layer that not only protects the titanium from oxidation but also significantly improves the electrode's performance and longevity.
Let's delve deeper into the specific aspects of these anodes to understand their advantages, lifespan, and industrial applications.
What are the advantages of Ruthenium Iridium Coated Titanium Anodes?
Ruthenium Iridium Coated Titanium Anodes offer numerous advantages that make them the preferred choice in many electrochemical processes:
1. High Catalytic Activity: The mixed metal oxide coating of ruthenium and iridium provides exceptional catalytic properties. This high catalytic activity allows for efficient electron transfer reactions, reducing the energy required for electrochemical processes. As a result, these anodes can operate at lower overpotentials compared to traditional anodes, leading to significant energy savings in industrial applications.
2. Corrosion Resistance: The combination of the titanium substrate and the noble metal oxide coating creates an anode with superior corrosion resistance. This property is particularly valuable in aggressive environments, such as those containing chlorides or acidic solutions. The excellent corrosion resistance ensures that the anodes maintain their performance and structural integrity over extended periods, even under harsh operating conditions.
3. Low Oxygen Overpotential: Ruthenium Iridium Coated Titanium Anodes exhibit a remarkably low oxygen overpotential. This means that they require less energy to drive the oxygen evolution reaction, which is crucial in processes like chlorine production and water electrolysis. The reduced overpotential translates to lower operating costs and improved energy efficiency in industrial applications.
4. Dimensional Stability: Unlike some traditional anodes that may experience significant wear or dimensional changes during operation, Ruthenium Iridium Coated Titanium Anodes maintain their shape and size over time. This dimensional stability is essential for maintaining consistent performance and avoiding the need for frequent adjustments or replacements in industrial equipment.
5. Versatility: These anodes are suitable for a wide range of applications and can operate effectively in various electrolytes and pH conditions. Their versatility makes them valuable in diverse industries, from water treatment and metal recovery to chemical manufacturing and energy storage.
6. Low Chlorine Overpotential: In chlor-alkali processes, Ruthenium Iridium Coated Titanium Anodes demonstrate a low chlorine overpotential. This property is crucial for efficient chlorine production, as it allows for higher current densities and improved energy efficiency in the electrolysis of brine solutions.
These advantages collectively contribute to the growing popularity of Ruthenium Iridium Coated Titanium Anodes in various industrial applications, driving innovations in electrochemical processes and enabling more sustainable and efficient operations.
How long do Ruthenium Iridium Coated Titanium Anodes last?
The lifespan of Ruthenium Iridium Coated Titanium Anodes is a critical factor in their industrial application, as it directly impacts operational costs, maintenance schedules, and overall process efficiency. These anodes are renowned for their exceptional durability and longevity, but their exact lifespan can vary depending on several factors:
1. Operating Conditions: The specific conditions under which the anodes operate play a significant role in determining their lifespan. Factors such as current density, electrolyte composition, temperature, and pH can all affect the rate of wear on the oxide coating. Generally, anodes operating under moderate conditions can last anywhere from 5 to 10 years or even longer.
2. Current Density: The applied current density is one of the most critical factors affecting anode lifespan. Higher current densities typically lead to faster degradation of the oxide coating. However, Ruthenium Iridium Coated Titanium Anodes are designed to withstand relatively high current densities, often operating efficiently at 3-10 kA/m² or higher, depending on the specific application.
3. Electrolyte Composition: The composition of the electrolyte solution can significantly impact anode longevity. Chloride-containing electrolytes, for example, can be particularly aggressive. However, the ruthenium-iridium oxide coating provides excellent resistance to chlorine evolution, making these anodes suitable for long-term use in chlor-alkali processes.
4. Maintenance Practices: Proper maintenance can substantially extend the life of these anodes. Regular inspection, cleaning, and adherence to recommended operating parameters can help prevent premature degradation. Some facilities implement rotation schedules for anodes to ensure even wear and maximize overall lifespan.
5. Coating Thickness and Composition: The thickness and exact composition of the ruthenium-iridium oxide coating influence the anode's lifespan. Thicker coatings generally last longer, but there's a trade-off with initial cost and performance characteristics. Some manufacturers offer customized coating compositions tailored to specific applications, optimizing the balance between performance and longevity.
It's important to note that while Ruthenium Iridium Coated Titanium Anodes have a finite lifespan, they often outlast many alternative anode materials. In some cases, these anodes have been reported to remain operational for 15-20 years under optimal conditions and proper maintenance.
The end of an anode's useful life is typically determined by a significant increase in cell voltage or a decrease in current efficiency, indicating that the oxide coating has degraded to a point where replacement is necessary. However, some facilities choose to recoat their anodes rather than replace them entirely, further extending their operational life.
Manufacturers and suppliers of Ruthenium Iridium Coated Titanium Anodes often provide specific lifetime estimates based on the intended application and operating conditions. These estimates can be valuable for planning maintenance schedules and budgeting for replacements. However, actual performance may vary, and many users find that careful operation and maintenance can lead to lifespans that exceed initial projections.
The long lifespan of these anodes, combined with their high performance and efficiency, contributes significantly to their cost-effectiveness in industrial applications. While the initial investment may be higher compared to some traditional anode materials, the extended operational life and reduced maintenance requirements often result in lower total cost of ownership over time.
Where are Ruthenium Iridium Coated Titanium Anodes used in industry?
Ruthenium Iridium Coated Titanium Anodes find widespread use across various industries due to their exceptional performance, durability, and versatility. Their unique properties make them invaluable in numerous electrochemical processes. Let's explore some of the key industrial applications where these anodes play a crucial role:
1. Swimming pool disinfection: The ruthenium iridium coated titanium anodes are designed to effectively disinfect the swimming pool in a safe, simple, and low-cost manner. The titanium electrode works grounded on the electrolysis process. When the electrode is connected to a power source, the catalyst coating reacts with water to produce hypochlorous acid and sodium hypochlorite, which effectively kill bacteria, contagions, and other microorganisms that can pollute pool water.
2. Electrolytic water treatment: For electrolytic water treatment, ruthenium iridium coated titanium anodes enable robust and reliable performance in electro-oxidation processes for wastewater treatment, disinfection, and organic contaminant destruction. The anode offers an electrocatalytic activity for the oxygen evolution reaction, which generates reactive oxidants from water to oxidize pollutants. The electrodes also withstand harsh acidic conditions commonly used during electrolytic water treatment.
3. Chlor-alkali production: In chlor-alkali production, ruthenium iridium coated titanium anodes allow durable and efficient performance for the electrolysis of brine solutions to produce chlorine and sodium hydroxide. The anodes’electrocatalytic properties coupled with their corrosion resistance in harsh brine electrolytes make them well-suited to withstand aggressive chlor-alkali process conditions for a long time.
4. Appliance&electronics: Ruthenium iridium electrodes can also be used in household appliances by electrolyzing water for sterilization and disinfection, as well as changing the pH of water to produce acidic or alkaline water.
The versatility of Ruthenium Iridium Coated Titanium Anodes is evident in their wide-ranging industrial applications. Their ability to perform efficiently in various electrolytes, resist corrosion, and maintain stable performance over long periods makes them an invaluable component in many electrochemical processes. As industries continue to seek more efficient and sustainable technologies, the role of these advanced anodes is likely to expand further, potentially opening up new applications in emerging fields such as carbon capture and utilization, advanced materials processing, and next-generation energy systems.
In conclusion, Ruthenium Iridium Coated Titanium Anodes represent a significant advancement in electrode technology. Their unique combination of high catalytic activity, corrosion resistance, and longevity makes them indispensable in a wide array of industrial applications. From chlorine production and water treatment to metal recovery and energy storage, these anodes continue to drive innovation and efficiency in electrochemical processes. As industries strive for more sustainable and energy-efficient operations, the importance of Ruthenium Iridium Coated Titanium Anodes is likely to grow, paving the way for new applications and technological advancements in the field of electrochemistry.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
2. Cardarelli, F. (2008). Materials handbook: a concise desktop reference. Springer Science & Business Media.
3. Kraft, A. (2007). Doped diamond: a compact review on a new, versatile electrode material. Int. J. Electrochem. Sci, 2(5), 355-385.
4. Comninellis, C., & Chen, G. (Eds.). (2010). Electrochemistry for the Environment. Springer Science & Business Media.
5. Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
6. Ribeiro, J., & De Andrade, A. R. (2004). Characterization of RuO2-Ta2O5 coated titanium electrode microstructure, morphology, and electrochemical investigation. Journal of The Electrochemical Society, 151(10), D106.
7. Chen, X., Chen, G., & Yue, P. L. (2001). Stable Ti/IrOx-Sb2O5-SnO2 anode for O2 evolution with high oxygen evolution potential. The Journal of Physical Chemistry B, 105(20), 4623-4628.
8. Duby, P. (1993). The history of progress in dimensionally stable anodes. Jom, 45(3), 41-43.
9. Shrivastava, P., & Moats, M. S. (2009). Wet film application techniques and their effects on the stability of RuO2-TiO2 coated titanium anodes. Journal of Applied Electrochemistry, 39(1), 107-116.
10. Menzel, N., Ortel, E., Mette, K., Kraehnert, R., & Strasser, P. (2013). Dimensionally stable Ru/Ir/TiO2-anodes with tailored mesoporosity for efficient electrochemical chlorine evolution. ACS Catalysis, 3(6), 1324-1333.
Related Industry Knowledge
- How Durable are MMO Wire Anodes?
- What Environments Are MMO Tubular Anodes Suitable For?
- What Are the Benefits of MMO Canistered Wire Anodes?
- How Do MMO Anodes Work In Different Environments?
- What is the Impact of Titanium Electrodes on the Marine Environment?
- What are the Environmental Benefits of Using Titanium Electrodes for Cobalt Electrowinning?
- Why Should You Consider Titanium Electrodes for Copper Plating?