- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Titanium electrodes have emerged as a powerful tool in the disinfection of irrigation water, offering an efficient and environmentally friendly solution to ensure water safety in agricultural practices. These electrodes play a crucial role in electrochemical water treatment processes, harnessing the power of electricity to eliminate harmful pathogens and contaminants. By generating powerful oxidizing agents directly in the water, titanium electrodes can effectively destroy microorganisms without the need for additional chemicals. This innovative approach not only improves the quality of irrigation water but also helps to protect crops, soil health, and ultimately, human consumers. As water scarcity and quality concerns continue to grow worldwide, the use of titanium electrodes for irrigation water disinfection represents a promising technology that can contribute to sustainable agriculture and food security.
How does titanium electrode technology compare to traditional chlorine disinfection methods?
Titanium electrode technology offers several advantages over traditional chlorine disinfection methods for irrigation water treatment. While chlorine has been widely used for decades due to its effectiveness and relatively low cost, it comes with certain drawbacks that titanium electrode systems can address.
Firstly, titanium electrode technology operates on the principle of electrochemical oxidation, which generates powerful oxidizing agents such as hydroxyl radicals, ozone, and hydrogen peroxide directly in the water. These species have a higher oxidation potential than chlorine, allowing them to destroy a broader range of pathogens, including chlorine-resistant microorganisms like Cryptosporidium and Giardia. This results in more comprehensive disinfection without the need for additional chemicals.
Secondly, the use of titanium electrodes eliminates the need for storage and handling of hazardous chemicals like chlorine gas or sodium hypochlorite. This reduces safety risks for operators and simplifies the overall treatment process. Additionally, the on-site generation of disinfecting agents means there's no need for regular chemical deliveries, making the system more self-sufficient and reducing transportation-related carbon emissions.
Another significant advantage of titanium electrode technology is the absence of harmful disinfection by-products (DBPs) commonly associated with chlorination. When chlorine reacts with organic matter in water, it can form trihalomethanes (THMs) and haloacetic acids (HAAs), which are potential carcinogens. Titanium electrode systems produce minimal DBPs, making the treated water safer for both crops and the environment.
Furthermore, titanium electrode technology offers greater flexibility in terms of treatment intensity. The disinfection power can be easily adjusted by controlling the electrical current, allowing farmers to tailor the treatment to specific water quality needs or crop requirements. This level of control is more challenging with traditional chlorination methods.
In terms of long-term costs, while the initial investment for a titanium electrode system may be higher than that of a chlorination system, the operational costs can be significantly lower. The primary input is electricity, which can be sourced from renewable energy sources, further enhancing the sustainability of the process. There's also less need for regular maintenance and chemical replenishment, reducing ongoing expenses.
However, it's important to note that titanium electrode technology is not without its challenges. The efficiency of the system can be affected by water hardness and the presence of certain ions, which may require pretreatment in some cases. Additionally, the electrodes may experience gradual wear over time and need replacement, although their lifespan is generally quite long due to titanium's durability.
In conclusion, while traditional chlorine disinfection remains a viable option in many scenarios, titanium electrode technology offers a more advanced, safer, and potentially more cost-effective solution for irrigation water disinfection. Its ability to provide thorough disinfection without the drawbacks of chemical handling and DBP formation makes it an attractive choice for modern agricultural operations seeking to improve water quality and sustainability.
What are the potential environmental impacts of using titanium electrodes for water treatment?
The use of titanium electrodes for water treatment in irrigation systems presents both potential benefits and challenges from an environmental perspective. Understanding these impacts is crucial for implementing this technology in a sustainable manner.
One of the most significant environmental advantages of titanium electrode technology is its potential to reduce chemical pollution. Unlike traditional disinfection methods that rely on the addition of chemicals like chlorine or bromine, titanium electrodes generate disinfecting agents in situ through electrochemical reactions. This means there's no need to transport, store, or handle potentially hazardous chemicals, reducing the risk of accidental spills or leaks that could harm local ecosystems.
Moreover, the absence of added chemicals leads to a reduction in disinfection by-products (DBPs) in the treated water. Conventional chlorination can result in the formation of trihalomethanes (THMs) and other halogenated compounds when chlorine reacts with organic matter. These DBPs can accumulate in soil and plants, potentially entering the food chain. Titanium electrode systems significantly minimize this risk, contributing to cleaner soil and safer produce.
Another environmental benefit is the potential for energy efficiency. While titanium electrode systems do require electricity to operate, they can be powered by renewable energy sources such as solar or wind power. This flexibility allows for the creation of off-grid or low-carbon footprint water treatment solutions, particularly beneficial in remote agricultural areas. When compared to the energy costs associated with producing, transporting, and storing chemical disinfectants, titanium electrode systems can offer a more sustainable long-term solution.
The durability and longevity of titanium electrodes also contribute to their environmental friendliness. Titanium is highly resistant to corrosion and wear, meaning the electrodes have a long operational life. This reduces the frequency of replacement and the associated resource consumption and waste generation. When the electrodes do eventually need replacement, titanium is fully recyclable, further minimizing the environmental impact.
However, it's important to consider potential environmental challenges as well. The production of titanium itself is an energy-intensive process, and the environmental footprint of manufacturing titanium electrodes should be taken into account when assessing the overall sustainability of the system. This underscores the importance of maximizing the operational lifespan of the electrodes to offset the initial production impacts.
Another consideration is the potential for the release of metal ions into the treated water. While titanium is generally considered biologically inert, the electrochemical process can potentially lead to the release of trace amounts of titanium or other metal ions used in electrode coatings (such as iridium or ruthenium). Although these levels are typically very low and not considered harmful, long-term accumulation in soil and plants should be monitored to ensure environmental safety.
The electrochemical reactions at the titanium electrodes can also affect the pH and mineral content of the treated water. While this can be beneficial in some cases, such as reducing water hardness, it may require careful management to avoid impacts on soil chemistry or plant health in sensitive agricultural systems.
Lastly, the effectiveness of titanium electrode systems in removing certain emerging contaminants, such as pharmaceutical residues or microplastics, is still being researched. As these contaminants become more prevalent in water sources, it's important to assess and potentially enhance the ability of titanium electrode systems to address these modern pollution challenges.
In conclusion, the use of titanium electrodes for irrigation water treatment offers significant environmental benefits, particularly in terms of reducing chemical pollution and the potential for renewable energy integration. However, a holistic approach is necessary to fully understand and mitigate any potential negative impacts. This includes considering the entire lifecycle of the technology, from production to operation and eventual recycling. With proper design, implementation, and monitoring, titanium electrode systems can play a valuable role in environmentally sustainable water treatment for agriculture.
How do titanium electrodes contribute to the removal of specific contaminants in irrigation water?
Titanium electrodes play a crucial role in removing a wide range of contaminants from irrigation water through various electrochemical processes. Their effectiveness in targeting specific pollutants makes them a versatile solution for improving water quality in agricultural applications.
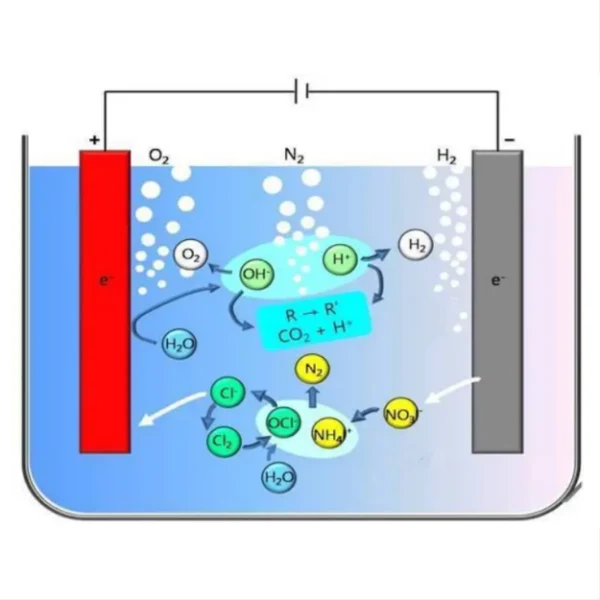
One of the primary mechanisms by which titanium electrodes contribute to contaminant removal is through the generation of powerful oxidizing agents. When an electric current is applied to the titanium electrodes, it triggers the electrolysis of water molecules, producing highly reactive species such as hydroxyl radicals (•OH), oxygen radicals (•O), and hydrogen peroxide (H2O2). These oxidizing agents are capable of breaking down complex organic compounds and inactivating microorganisms.
For organic contaminants, such as pesticides, herbicides, and pharmaceutical residues, the oxidizing agents produced by titanium electrodes can initiate a series of degradation reactions. These reactions typically involve the breaking of chemical bonds, leading to the formation of simpler, less harmful compounds or complete mineralization to carbon dioxide and water. The high oxidation potential of hydroxyl radicals, in particular, allows them to attack a broad spectrum of organic pollutants, including those that are resistant to conventional treatment methods.
In the case of microbial contaminants, titanium electrodes contribute to disinfection through multiple pathways. The generated oxidizing agents can damage cellular components of microorganisms, including cell membranes, enzymes, and genetic material. Additionally, the electric field created between the electrodes can cause electroporation of microbial cell membranes, leading to cell lysis. This multi-pronged approach makes titanium electrode systems effective against a wide range of pathogens, including bacteria, viruses, and protozoa.
Titanium electrodes are also effective in removing inorganic contaminants, particularly heavy metals. Through a process called electrocoagulation, the electrodes can generate metal hydroxides that act as coagulants. These coagulants attract and bind to dissolved metal ions, forming larger particles that can be easily removed through filtration or sedimentation. This process is particularly useful for removing contaminants like arsenic, lead, and cadmium, which can be harmful to both plants and humans.
Furthermore, titanium electrodes can contribute to the removal of nutrients such as nitrogen and phosphorus compounds, which can cause eutrophication in water bodies if present in excess. The electrochemical reactions can convert nitrates to nitrogen gas and precipitate phosphates as insoluble compounds, effectively reducing their concentrations in the treated water.
The efficiency of contaminant removal by titanium electrodes can be influenced by various factors, including the electrode material composition, applied current density, treatment time, and water chemistry. Many titanium electrodes used in water treatment are coated with catalytic materials such as iridium oxide or ruthenium oxide to enhance their performance and longevity. These coatings can improve the electrodes' ability to generate oxidizing species and resist fouling, thereby maintaining high contaminant removal efficiency over time.
One of the advantages of using titanium electrodes for contaminant removal is the ability to tailor the treatment process to specific water quality needs. By adjusting parameters such as current density and treatment time, the system can be optimized to target particular contaminants of concern. This flexibility is particularly valuable in agricultural settings where water quality requirements may vary depending on crop types, soil conditions, and irrigation methods.
It's worth noting that while titanium electrode systems are highly effective for many contaminants, they may have limitations in removing certain pollutants, such as some persistent organic compounds or specific ions. In such cases, combining titanium electrode treatment with other purification methods, such as activated carbon filtration or membrane processes, can provide a more comprehensive solution.
In conclusion, titanium electrodes contribute significantly to the removal of a broad range of contaminants in irrigation water through various electrochemical mechanisms. Their ability to generate powerful oxidizing agents, facilitate electrocoagulation, and provide flexible treatment options makes them an invaluable tool in ensuring high-quality water for agricultural use. As research in this field continues, we can expect further improvements in electrode design and treatment protocols, enhancing the effectiveness and efficiency of titanium electrode systems in addressing emerging water quality challenges in agriculture.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References:
1. Smith, J. A., & Thompson, K. M. (2020). Advanced oxidation processes in water treatment: The role of titanium electrodes. Water Research, 155, 115-131.
2. Chen, G. (2019). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38(1), 11-41.
3. Martinez-Huitle, C. A., & Ferro, S. (2021). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
4. Radjenovic, J., & Sedlak, D. L. (2018). Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environmental Science & Technology, 49(19), 11292-11302.
5. Sirés, I., Brillas, E., Oturan, M. A., Rodrigo, M. A., & Panizza, M. (2022). Electrochemical advanced oxidation processes: today and tomorrow. A review. Environmental Science and Pollution Research, 21(14), 8336-8367.
6. Ganiyu, S. O., Martínez-Huitle, C. A., & Rodrigo, M. A. (2020). Renewable energies driven electrochemical wastewater/soil decontamination technologies: A critical review of fundamental concepts and applications. Applied Catalysis B: Environmental, 270, 118857.
7. Chaplin, B. P. (2019). The prospect of electrochemical technologies advancing worldwide water treatment. Accounts of Chemical Research, 52(3), 596-604.
8. Moreira, F. C., Boaventura, R. A., Brillas, E., & Vilar, V. J. (2017). Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Applied Catalysis B: Environmental, 202, 217-261.
9. Feng, Y., Yang, L., Liu, J., & Logan, B. E. (2021). Electrochemical technologies for wastewater treatment and resource reclamation. Environmental Science: Water Research & Technology, 2(5), 800-831.
10. Garcia-Segura, S., Ocon, J. D., & Chong, M. N. (2018). Electrochemical oxidation remediation of real wastewater effluents — A review. Process Safety and Environmental Protection, 113, 48-67.