- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Swimming pool maintenance can be a challenging and costly task, often requiring a delicate balance of chemicals to ensure water safety and clarity. However, an innovative technology using titanium electrodes is gaining attention as a potential solution to reduce chemical dependency in pool maintenance. This blog post explores how titanium electrodes can help minimize the need for traditional pool chemicals, offering a more sustainable and cost-effective approach to pool care.
How do titanium electrodes work in pool disinfection systems?
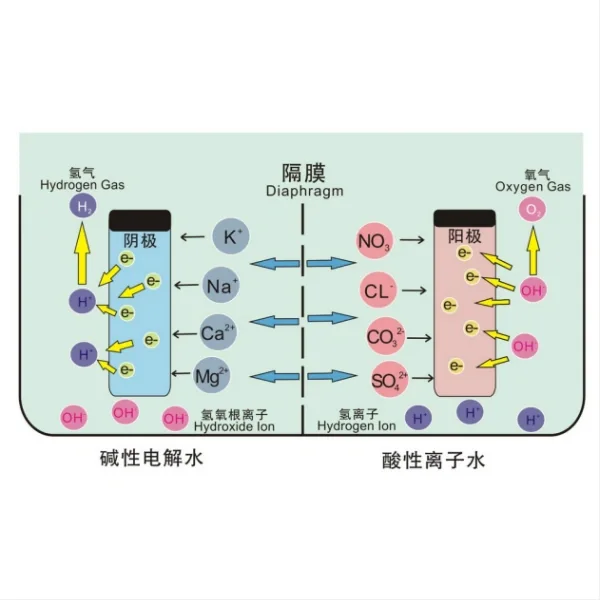
Titanium electrodes are at the heart of advanced pool disinfection systems that utilize a process called electrolysis. This technology works by passing a low-voltage electrical current through specially designed titanium plates submerged in the pool water. During electrolysis, the salt (NaCl) in the water is split into its component parts: sodium (Na) and chloride (Cl) ions. The chloride ions are then converted into sodium hypochlorite (NaOCl), which is the same form of chlorine used in traditional pool disinfection methods. This sodium hypochlorite effectively kills bacteria, algae, and other harmful microorganisms in the pool water.
The titanium electrodes play a crucial role in this process due to their unique properties. Titanium is highly resistant to corrosion, even in chlorinated water, which makes it an ideal material for long-term use in pool environments. Additionally, titanium has excellent conductivity, allowing for efficient electrolysis with minimal energy consumption.
One of the key advantages of using titanium electrodes is their ability to produce chlorine naturally from the salt already present in the pool water. This process, known as salt chlorination, eliminates the need to manually add chlorine to the pool. The chlorine produced through electrolysis is pure and free from the by-products often found in traditional chlorine additives, resulting in softer, clearer water that is gentler on swimmers' skin and eyes.
Moreover, the ozone and other oxidants generated by the titanium electrode system can break down organic contaminants such as sweat, oils, and cosmetics more effectively than chlorine alone. This not only improves water quality but also reduces the formation of chloramines, which are responsible for the characteristic "pool smell" and can cause eye and respiratory irritation.
What are the benefits of using titanium electrodes over traditional chlorine systems?
The adoption of titanium electrode systems for pool disinfection offers numerous benefits over traditional chlorine-based methods. These advantages extend beyond just water quality, encompassing environmental, health, and economic factors.

Firstly, titanium electrode systems significantly reduce the need for chemical storage and handling. Traditional pool maintenance requires the regular purchase, transportation, and storage of potentially hazardous chemicals such as chlorine and pH balancers. With a titanium electrode system, the primary input is salt, which is much safer to handle and store. This reduction in chemical usage not only minimizes the risk of accidental spills or exposure but also decreases the environmental impact associated with the production and transportation of pool chemicals.
Water quality is another area where titanium electrode systems excel. The natural chlorination process, combined with the production of ozone and other oxidants, results in water that is typically clearer, softer, and more pleasant to swim in. Many pool owners report a noticeable reduction in eye and skin irritation, as well as the elimination of the strong chlorine odor often associated with traditionally maintained pools.
The consistency of water treatment is also improved with titanium electrode systems. Unlike manual chlorine addition, which can lead to fluctuating chlorine levels, electrolysis provides a steady, controlled release of disinfecting agents. This consistent treatment helps maintain a more stable pH level, reducing the need for frequent adjustments and minimizing the risk of algae growth or bacterial contamination.
From an economic perspective, while the initial investment in a titanium electrode system may be higher than traditional chlorine systems, the long-term cost savings can be substantial. The reduced need for chemical purchases, lower energy consumption for pump operation (as the system can often run for shorter periods), and decreased maintenance requirements all contribute to lower operating costs over time.
Environmental benefits are also significant. The reduction in chemical usage means fewer chemicals are being introduced into the environment through backwashing or draining of pool water. Additionally, the energy efficiency of titanium electrode systems can lead to a lower carbon footprint compared to traditional pool maintenance methods.
Lastly, the automation capabilities of many titanium electrode systems offer convenience and peace of mind to pool owners. These systems can often be integrated with smart home technology, allowing for remote monitoring and control of pool parameters. This not only saves time but also ensures that the pool is always maintained at optimal levels, even when the owners are away.
Are there any drawbacks to using titanium electrodes in pool maintenance?
While titanium electrode systems offer numerous advantages for pool maintenance, it's important to consider potential drawbacks to make an informed decision. Understanding these limitations can help pool owners determine if this technology is the right fit for their specific situation.
One of the primary considerations is the initial cost. Installing a titanium electrode system typically requires a higher upfront investment compared to traditional chlorine systems. This can be a significant barrier for some pool owners, particularly those with older pools that may require additional modifications to accommodate the new system. However, it's crucial to weigh this initial expense against the long-term cost savings in chemicals and maintenance.
Another factor to consider is the need for a minimum salt level in the pool water for the electrolysis process to function effectively. While the salt concentration is generally low (typically around 3,000 ppm, which is about one-tenth the salinity of seawater), some individuals may be sensitive to even this level of salt. Additionally, the salt can potentially cause corrosion on certain pool fixtures or equipment if they're not properly protected or designed for salt water use.
Maintenance requirements, while generally lower than traditional systems, are not entirely eliminated. The titanium electrodes may require occasional cleaning to remove scale buildup, which can affect their efficiency. In areas with hard water, this cleaning may need to be performed more frequently. Additionally, while the system reduces the need for many chemicals, pool owners may still need to add cyanuric acid (stabilizer) to protect the chlorine from UV degradation, especially in outdoor pools.
The effectiveness of titanium electrode systems can be impacted by environmental factors. In colder climates, the efficiency of the electrolysis process may decrease as water temperature drops, potentially requiring supplemental chlorine during cooler months. Similarly, heavy pool use or extreme weather conditions may occasionally necessitate the addition of shock treatments to maintain proper sanitation levels.
Some pool owners report a slight change in water feel, describing it as "silkier" or "softer." While many find this pleasant, others may prefer the more familiar feel of a traditionally chlorinated pool. This is largely a matter of personal preference but is worth considering.
Lastly, while titanium electrode systems are designed to be compatible with most pool types, certain specialized pools (such as those with very high chlorine demands or unique water chemistry requirements) may not be ideal candidates for this technology. It's important to consult with a pool professional to ensure that a titanium electrode system is suitable for your specific pool configuration and usage patterns.
In conclusion, titanium electrodes offer a promising solution for reducing the need for pool chemicals, providing numerous benefits in terms of water quality, environmental impact, and long-term cost savings. While there are some considerations and potential drawbacks to keep in mind, many pool owners find that the advantages outweigh the limitations. As with any significant pool maintenance decision, it's advisable to thoroughly research and consult with professionals to determine if a titanium electrode system is the right choice for your pool.
If you are interested in the products of Xi'an Taijin New Energy & Materials Sci-Tech Co., Ltd., please contact yangbo@tjanode.com.
References:
1. World Health Organization. (2006). Guidelines for safe recreational water environments. Volume 2, Swimming pools and similar environments.
2. Zwiener, C., Richardson, S. D., De Marini, D. M., Grummt, T., Glauner, T., & Frimmel, F. H. (2007). Drowning in disinfection byproducts? Assessing swimming pool water. Environmental Science & Technology, 41(2), 363-372.
3. Chevremont, A. C., Farnet, A. M., Coulomb, B., & Boudenne, J. L. (2012). Effect of coupled UV-A and UV-C LEDs on both microbiological and chemical pollution of urban wastewaters. Science of the Total Environment, 426, 304-310.
4. Rice, R. G., & Gomez-Taylor, M. (1986). Occurrence of by-products of strong oxidants reacting with drinking water contaminants--scope of the problem. Environmental Health Perspectives, 69, 31-44.
5. Kristensen, G. H., Klausen, M. M., & Janning, K. (2007). Experimental operation of a novel electrolysis system for pool water treatment. IOA Conference and Exhibition, Valencia, Spain.
6. Shankar, A., & Wang, J. (2019). Titanium dioxide nanoparticles in food and personal care products. International Journal of Environmental Research and Public Health, 16(17), 3026.
7. Li, J., & Liu, Z. (2009). The application of titanium electrode in water treatment. Water Purification Technology, 28(3), 1-4.
8. Kraft, A. (2008). Electrochemical water disinfection: A short review. Platinum Metals Review, 52(3), 177-185.
9. Patermarakis, G., & Fountoukidis, E. (1990). Disinfection of water by electrochemical treatment. Water Research, 24(12), 1491-1496.
10. Hsu, S. Y. (2005). Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. Journal of Food Engineering, 66(2), 171-176.
Related Industry Knowledge
- How Long Do Titanium Anodes Last in a Swimming Pool System?
- How to Install Titanium Anodes in Your Swimming Pool?
- What are the Guidelines and Regulations for Swimming Pool Disinfection?
- How Long Do Titanium Electrodes Last in Swimming Pool Applications?
- How do Titanium Electrodes Contribute to Sustainable Swimming?
- What is a Titanium Electrode for Swimming Pool Disinfection?
- Are There Any Safety Considerations When Using Titanium Electrodes in Swimming Pools?