- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
Titanium coated with Ruthenium Iridium is a specialized material used in various industrial applications, particularly in electrochemistry and water treatment. This coating combines the durability of titanium with the catalytic properties of ruthenium and iridium, creating a highly effective and long-lasting electrode material. The ruthenium-iridium coating enhances the titanium's surface properties, making it more resistant to corrosion and improving its electrochemical performance. This innovative material is often referred to as Ruthenium Iridium coated Titanium Anodes, which are widely used in chlorine production, water electrolysis, and other electrochemical processes.
What are the advantages of using Ruthenium Iridium coated Titanium Anodes?
Ruthenium Iridium coated Titanium Anodes offer several significant advantages in various industrial applications. These anodes combine the strength and lightweight properties of titanium with the exceptional catalytic activity of ruthenium and iridium, resulting in a superior electrode material.
One of the primary advantages is the enhanced durability and longevity of these anodes. The ruthenium-iridium coating provides excellent protection against corrosion, even in highly aggressive environments. This resistance to corrosion allows the anodes to maintain their performance over extended periods, reducing the need for frequent replacements and minimizing downtime in industrial processes.
Another key benefit is the improved electrocatalytic activity of Ruthenium Iridium coated Titanium Anodes. The coating significantly lowers the overpotential required for various electrochemical reactions, leading to increased energy efficiency. This is particularly important in applications such as chlorine production and water electrolysis, where energy consumption is a major cost factor.
The versatility of these anodes is also noteworthy. They can be used in a wide range of electrolytes and pH conditions, making them suitable for diverse industrial processes. From seawater chlorination to wastewater treatment, these anodes have proven their effectiveness across multiple applications.
Furthermore, Ruthenium Iridium coated Titanium Anodes exhibit excellent dimensional stability. Unlike some other electrode materials that may deform or erode over time, these anodes maintain their shape and surface characteristics, ensuring consistent performance throughout their lifespan.
The combination of these advantages makes Ruthenium Iridium coated Titanium Anodes a cost-effective solution for many industries. While the initial investment may be higher compared to some traditional electrode materials, the long-term benefits in terms of durability, efficiency, and reduced maintenance often result in significant cost savings over time.
How are Ruthenium Iridium coated Titanium Anodes manufactured?
The manufacturing process of Ruthenium Iridium coated Titanium Anodes is a sophisticated procedure that requires precision and expertise. The process typically involves several key steps to ensure the production of high-quality anodes with optimal performance characteristics.
The first step in the manufacturing process is the preparation of the titanium substrate. High-grade titanium sheets or mesh are carefully selected and cleaned to remove any surface contaminants. This cleaning process often involves both mechanical and chemical treatments to ensure a pristine surface for coating adhesion.
Once the titanium substrate is prepared, the next step is the application of the ruthenium-iridium coating. This is typically done through a thermal decomposition method. In this process, precursor solutions containing ruthenium and iridium compounds are applied to the titanium surface. The coated substrate is then subjected to controlled heating cycles, which decompose the precursor compounds and form a stable oxide layer on the titanium surface.
The coating process may involve multiple layers to achieve the desired thickness and composition. Each layer is carefully applied and heat-treated before the next layer is added. This multi-layer approach helps to ensure a uniform and durable coating.
After the coating process, the anodes undergo a series of quality control checks. These may include visual inspections, thickness measurements, and electrochemical tests to verify the coating's integrity and performance. Advanced techniques such as scanning electron microscopy (SEM) and X-ray diffraction (XRD) may be used to analyze the coating structure and composition.
The final steps in the manufacturing process involve the assembly of the anodes into their final form. This may include attaching current distributors, insulating parts of the anode not intended for electrochemical activity, and packaging for shipment.
It's important to note that the exact manufacturing process can vary depending on the specific application requirements and the manufacturer's proprietary techniques. Some manufacturers may use variations of this process or incorporate additional steps to enhance certain properties of the anodes.
The quality of the manufacturing process directly impacts the performance and longevity of the anodes. Factors such as the purity of the precursor materials, the precision of the coating application, and the control of the heat treatment process all play crucial roles in determining the final quality of the Ruthenium Iridium coated Titanium Anodes.
What are the main applications of Ruthenium Iridium coated Titanium Anodes?
Ruthenium Iridium coated Titanium Anodes find applications across a wide range of industries due to their unique properties and performance characteristics. These anodes have become integral components in numerous electrochemical processes and water treatment applications.
One of the primary applications of these anodes is in chlorine production. In the chlor-alkali industry, Ruthenium Iridium coated Titanium Anodes are used for the electrolysis of sodium chloride (NaCl) solutions to produce chlorine gas, sodium hydroxide, and hydrogen. The high catalytic activity of the ruthenium-iridium coating allows for efficient chlorine evolution, while the durability of the titanium substrate ensures long-term operation in the corrosive environment of chlorine cells.
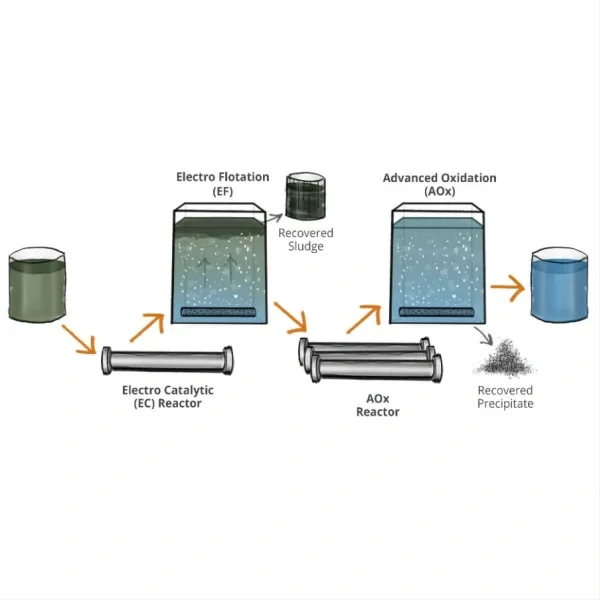
Water and wastewater treatment represent another significant area of application for these anodes. They are widely used in electrochemical advanced oxidation processes (EAOPs) for the removal of organic pollutants from water. The anodes' ability to generate powerful oxidizing species such as hydroxyl radicals makes them effective in breaking down complex organic compounds, including pharmaceuticals, pesticides, and industrial chemicals that are resistant to conventional treatment methods.
In the field of metal recovery and electroplating, Ruthenium Iridium coated Titanium Anodes play a crucial role. They are used in electrowinning processes to recover metals from solutions, as well as in various electroplating applications where precise control of the electrochemical process is required.
The anodes also find application in cathodic protection systems, particularly in marine environments. They are used to protect metal structures such as ship hulls, offshore platforms, and underwater pipelines from corrosion by impressing an electric current.
In the emerging field of water electrolysis for hydrogen production, Ruthenium Iridium coated Titanium Anodes are being explored as potential electrode materials. Their high catalytic activity and stability in acidic environments make them promising candidates for proton exchange membrane (PEM) electrolyzers.
The versatility of these anodes extends to other applications as well, including ozone generation for water disinfection, electrochemical synthesis of chemicals, and even in some biomedical applications for the modification of implant surfaces.
As research in electrochemistry and materials science continues to advance, new applications for Ruthenium Iridium coated Titanium Anodes are likely to emerge, further expanding their utility across various industries.
If you are interested in the products of Xi'an Taijin New Energy Technology Co., Ltd., please contact yangbo@tjanode.com.
References
1. Kraft, A. (2007). Doped diamond: A compact review on a new, versatile electrode material. International Journal of Electrochemical Science, 2, 355-385.
2. Trasatti, S. (2000). Electrocatalysis: understanding the success of DSA®. Electrochimica Acta, 45(15-16), 2377-2385.
3. Chen, X., Chen, G., & Yue, P. L. (2001). Stable Ti/RuO2–Sb2O5–SnO2 electrodes for O2 evolution. The Journal of Physical Chemistry B, 105(20), 4623-4628.
4. Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324-1340.
5. Comninellis, C., & Chen, G. (Eds.). (2010). Electrochemistry for the Environment. Springer Science & Business Media.
6. Panic, V. V., Dekanski, A. B., Milonjic, S. K., Atanasoski, R. T., & Nikolic, B. Z. (1999). RuO2–TiO2 coated titanium anodes obtained by the sol-gel procedure and their electrochemical behaviour in the chlorine evolution reaction. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 157(1-3), 269-274.
7. Hu, J. M., Zhang, J. Q., & Cao, C. N. (2004). Oxygen evolution reaction on IrO2-based DSA® type electrodes: kinetics analysis of Tafel lines and EIS. International Journal of Hydrogen Energy, 29(8), 791-797.
8. Daghetti, A., Lodi, G., & Trasatti, S. (1983). Interfacial properties of oxides used as anodes in the electrochemical technology. Materials Chemistry and Physics, 8(1), 1-90.
9. Xu, L., Xiao, Y., van Sandwijk, A., Xu, Q., & Yang, Y. (2015). Production of ruthenium and iridium coated titanium electrodes by thermal decomposition. Materials, 8(4), 1601-1612.
10. Cardarelli, F. (2008). Materials handbook: a concise desktop reference. Springer Science & Business Media.